Oncotarget recently published "Targeting breast cancer metabolism with a novel inhibitor of mitochondrial ATP synthesis" by Kim, et al. which reported that the authors show that CADD522 inhibits mitochondrial oxidative phosphorylation by decreasing the mitochondrial oxygen consumption rate and ATP production in human breast cancer cells in a RUNX2-independent manner.
The enzyme activity of mitochondrial ATP synthase was inhibited by CADD522 treatment.
Differential scanning fluorimetry also demonstrated interaction of α subunits of the F1-ATP synthase to CADD522. These results suggest that CADD522 might target the enzymatic F1 subunits in the ATP synthase complex.
CADD522 increased the levels of intracellular reactive oxygen species, which was prevented by MitoQ, a mitochondria-targeted antioxidant, suggesting that cancer cells exposed to CADD522 may elevate ROS from mitochondria.
Collectively, the Oncotarget author's data suggest in vitro proof-of-concept that supports inhibition of mitochondrial ATP synthase and ROS generation as contributors to the effectiveness of CADD522 in suppression of tumor growth.
"The Oncotarget author's data suggest in vitro proof-of-concept that supports inhibition of mitochondrial ATP synthase and ROS generation as contributors to the effectiveness of CADD522 in suppression of tumor growth"
Dr. Myoung Sook Kim from The University of Maryland School of Medicine and Dr. Antonino Passaniti also from The University of Maryland School of Medicine as well as The Marlene & Stewart Greenebaum Comprehensive Cancer Center and The Veteran's Health Administration Research & Development Service said, "Breast cancer (BC) is the second leading cause of cancer-related deaths among women, but early detection and improved treatment options have led to increased patient survival."
Resveratrol targets Complexes I and II, but it also targets the F1 domain of ATP synthase, resulting in a non-competitive inhibition of F1-ATP synthase activity.
Aurovertin B inhibits the activity of ATP synthase by interacting with the β subunit of the F1-ATP synthase and limits proliferation of BC cells with little influence on the normal mammary epithelial cells.
Citreoviridin, which is in the same class as aurovertin, targets the β subunit of the F1-ATP synthase, inhibits the growth and proliferation of BC cells as well as lung adenocarcinoma cells.
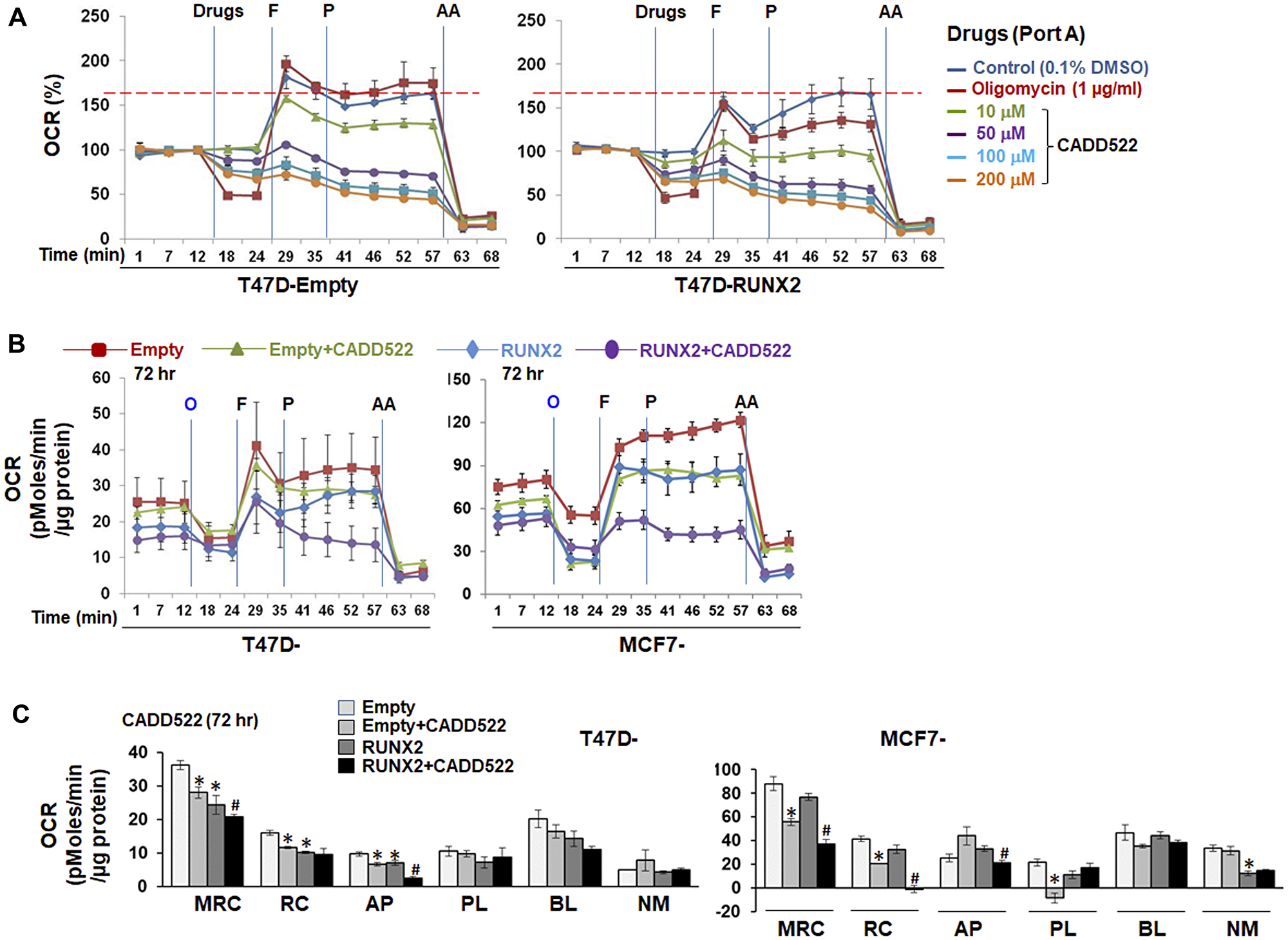
Figure 1: Inhibitory effect of CADD522 on mitochondrial OCR. (A) (A) Acute OCR changes were determined in T47D-RUNX2 and -Empty cells with no prior CADD522 treatment. Cells seeded in Seahorse plates were incubated overnight in growth medium without G418, and then replenished with Seahorse-Certified Medium on the assay day. Vehicle control (0.1% DMSO), Oligomycin or CADD522 were directly injected into port A of the XF Extracellular Flux Analyzer, and OCR was measured. Data (% OCR) are presented as the percentage of the basal OCR value at t = 12 min (100%). Experiments were performed in four replicates and repeated twice (mean ± SD). O, Oligomycin (1 μg/ml); F, FCCP (1 μM, port B); P, pyruvate (10 mM, port C); AA, antimycin A (1 μM, port D). Red line, maximal OCR value of the Control at t = 57 min. (B) Cells were treated with CADD522 for 6~72 hr and replenished with Seahorse-Certified Medium with no CADD522. OCR was measured in the XF Extracellular Flux Analyzer. Data (mean ± SD) are presented as OCR (pMoles/min/μg protein). (C) Mitochondrial respiratory parameters were calculated as described in Material and Methods: Maximal respiratory capacity (MRC), mitochondrial reserve capacity (RC), ATP production-linked respiration (AP), proton leak-linked respiration (PL), baseline respiration (BL), and non-mitochondrial respiration (NM). Experiments were performed in four replicates and data are expressed as mean ± SE from two independent experiments. *P < 0.05 compared to Empty cells with vehicle control (0.1% DMSO); #P < 0.05 compared to RUNX2-expressing cells with vehicle control.
Rhodamine123 inhibits BC colony formation, which was reported to be due to inhibition of the FoF1-ATP synthase enzyme complex leading to ATP depletion.
Unexpectedly, however, the authors observed that CADD522 directly targeted mitochondrial oxidative phosphorylation, decreasing mitochondrial OCR and ATP production in BC cells.
The Kim/Passaniti Research Team concluded in their Oncotarget Research paper that moreover, expression of the catalytic subunit β-F1-ATP synthase is tightly regulated by post-transcriptional mechanisms that affect mRNA localization, stability and translation.
The mRNA expression of ATP5B in MCF7 and MDA-468 cells was substantially decreased by CADD522 treatment.
These results indicate that CADD522 might inhibit ATP synthase activity through regulation of target gene expression levels and/or the activity of the catalytic β subunit of the mitochondrial F1-ATP synthase.
In summary, the findings demonstrate that mitochondrial ATP synthase inhibition may be a valid therapeutic approach and that one mechanism that drives sensitivity of BC cells to CADD522 is suppression of mitochondrial metabolism.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27743
Full text - https://www.oncotarget.com/article/27743/text/
Correspondence to - Myoung Sook Kim - [email protected] and Antonino Passaniti - [email protected]
Keywords - mitochondrial ATP synthase, oxygen consumption rate, ATP synthesis, reactive oxygen species
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



