Oncotarget published "Trends in oligomannosylation and α1,2-mannosidase expression in human cancers" which reported that aberrant protein glycosylation is a prominent cancer feature. While many tumour-associated glycoepitopes have been reported, advances in glycoanalytics continue to uncover new associations between glycosylation and cancer.
Firstly, the quantitative glycomics data obtained across 34 cancerous cell lines demonstrated that oligomannosylation is a pan-cancer feature spanning in a wide abundance range. In keeping with literature, these author's quantitative glycomics data of tumour and matching control tissues and new MALDI-MS imaging data of tissue microarrays showed a strong cancer-associated elevation of oligomannosylation in both basal cell and squamous cell skin cancer and colorectal cancer.
Finally, expression data from public cancer repositories indicated that several 1,2-mannosidases are regulated in tumour tissues suggesting that these glycan-processing enzymes may contribute to the cancer-associated modulation of oligomannosylation.
Dr. Rebeca Kawahara and Dr. Morten Thaysen-Andersen from The Macquarie University said, "Protein glycosylation, the addition of complex carbohydrates (glycans) to polypeptides, is a ubiquitous and energy-demanding post-translational modification important for a plethora of inter- and intracellular processes."
Protein glycosylation, the addition of complex carbohydrates (glycans) to polypeptides, is a ubiquitous and energy-demanding post-translational modification important for a plethora of inter- and intracellular processes
Asparagine -linked glycans display remarkable molecular heterogeneity despite being assembled from only few different monosaccharide building blocks including mannose, galactose, fucose, glucose, N-acetylglucosamine and N-acetylneuraminic acid. The template-less N-glycan biosynthesis begins in the endoplasmic reticulum by the transfer of immature Glc-capped glycan precursors to Asn residues located in consensus sequences of acceptor polypeptides and continues in the Golgi compartments.
Several ER- and Golgi-resident 1,2-mannosidases catalyze the successive M9-to-M5 glycan processing. Specifically, mannosyl-oligosaccharide 1,2--mannosidase 1A, mannosyl-oligosaccharide 1,2--mannosidase IB, endoplasmic reticulum mannosyl-oligosaccharide 1,2--mannosidase and mannosyl-oligosaccharide 1,2--mannosidase 1C are known to trim the outer 1,2-Man residues of nascent glycoproteins to eventually form M5 devoid of any 1,2-Man residues.
Despite forming a considerable part of the N-glycome, the mannose-terminating N-glycans comprising both the oligomannosidic- and paucimannosidic-type have received comparably less attention in cancer research. These authors recently used state-of-the-art glycomics to systematically document that paucimannosylation is an overlooked feature in many cancer types including in brain, blood, bladder, melanoma and non-melanoma skin, breast, hepatocellular carcinoma, lung, ovarian , gastric, colorectal and prostate cancer.
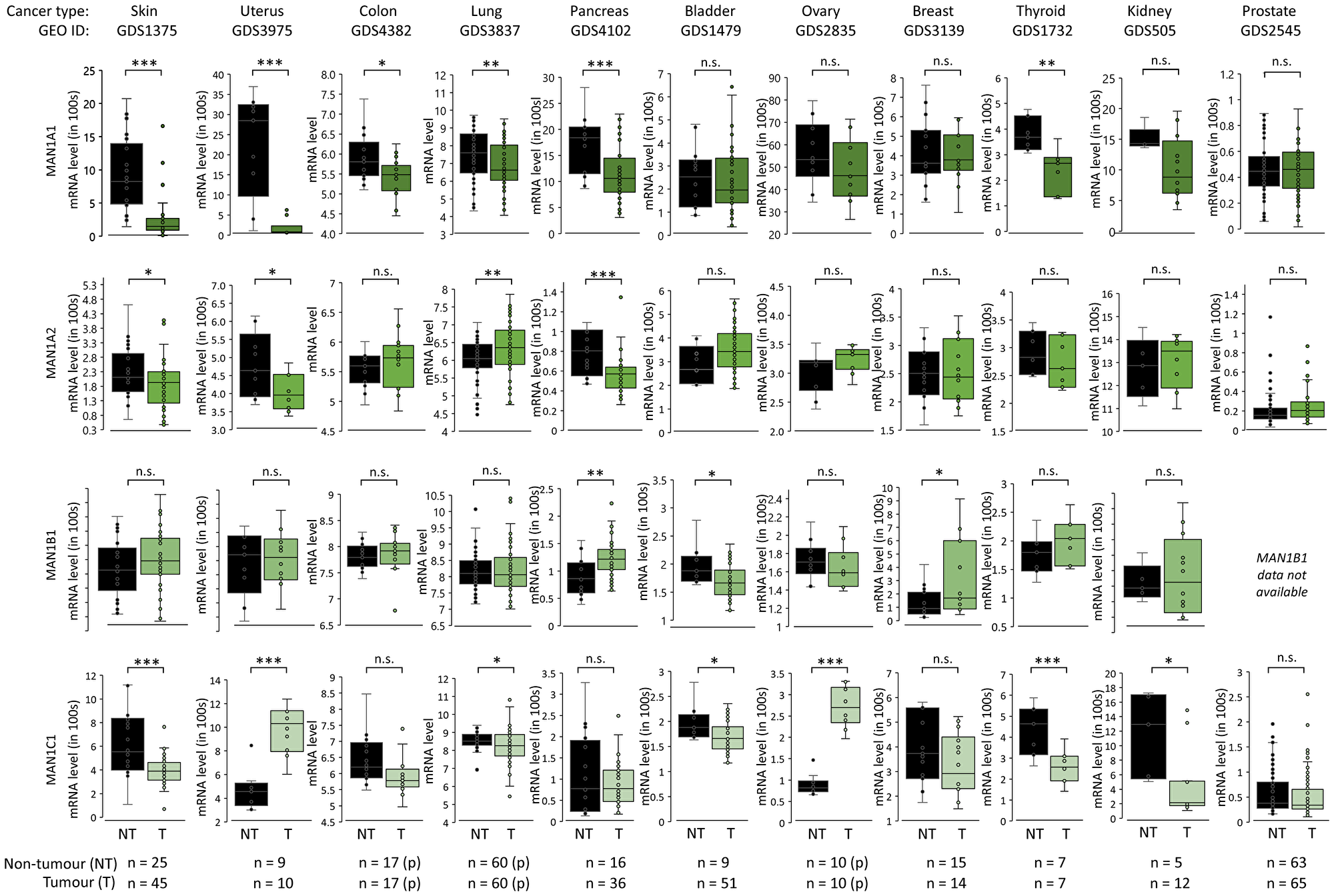
Figure 4: α1,2-mannosidase expression patterns in cancer. mRNA expression of MAN1A1, MAN1A2, MAN1B1 and MAN1C1 in paired (p) and unpaired tumour (different shading of green data points) and non-tumour (black data points) tissues across cancer types based on data extracted from the Gene Expression Omnibus (GEO). Statistics was performed using paired and unpaired two-tailed t-tests (n, sample numbers, as indicated below the graphs). *p < 0.05, **p < 0.001, ***p < 0.0001, n.s. not significant (p ≥ 0.05).
The Kawahara/Thaysen-Andersen Research Team concluded in their Oncotarget Research Output that for the lectin flow cytometry used to probe the cell surface oligomannosylation, concanavalin A is known to recognize not only mannosyl epitopes but also cross-react with other glycoepitopes including biantennary complex-type N-glycans and terminal glucose moieties [98].
While these authors showed that Me-α-Man competitively reduced ConA reactivity, the cell surface expression of oligomannosidic epitopes should therefore be confirmed using other mannose-recognizing lectins (ideally performed with and without endoglycosidase H treatment and α-Man competitors as appropriate controls) and/or using orthogonal methods including MS-based glycomics profiling after subcellular fractionation or cell surface capture or via lectin arrays [99] and should be studied more widely across other cancer cell lines to determine if cell surface expression of oligomannosylation is a general feature of cancer cells.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28064
Full text - https://www.oncotarget.com/article/28064/text/
Correspondence to - Rebeca Kawahara - [email protected] and Morten Thaysen-Andersen - [email protected]
Keywords - prostate cancer, castration resistance, detoxification, UDP-glucose dehydrogenase, patient-derived xenografts
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105