The cover for issue 35 of Oncotarget features Figure 4, "The in vivo pre- or post- topical application of BAY 11-7082 prevents the acidic bile-induced deregulation of cancer-related miRNA markers in 10-day exposed murine HM," by Vageli, et al. reported that Supraesophageal bile reflux at strongly acidic pH can cause hypopharyngeal squamous cell cancer, through activation of the oncogenic NF-κB-related pathway.
The authors hypothesize that topical pre- or post-application of pharmacologic NF-κB inhibitor, BAY 11-7082, on murine HM can effectively inhibit acidic bile induced oncogenic molecular events, similar to prior in vitro findings.
They demonstrate that the administration of BAY 11-7082, either before or after acidic bile, eliminates NF-κB activation, prevents overexpression of Bcl2, Rela, Stat3, Egfr, Tnf, Wnt5a, and deregulations of miR-192, miR-504, linked to bile reflux-related hypopharyngeal cancer.
Pre- but not post-application of NF-κB inhibitor, significantly blocks overexpression of Il6 and prostaglandin H synthases 2, and reverses miR-21, miR-155, miR-99a phenotypes, supporting its early bile-induced pro-inflammatory effect.
The authors thus provide novel evidence that topical administration of a pharmacological NF-κB inhibitor, either before or after acidic bile exposure can successfully prevent its oncogenic mRNA and miRNA phenotypes in HM, supporting the observation that co-administration of NF-κB inhibitor may not be essential in preventing early bile-related oncogenic events and encouraging a capacity for further translational exploration.
Dr. Clarence T. Sasaki from The Yale Larynx Laboratory, Department of Surgery at The Yale School of Medicine in New Haven, Connecticut said, "Tobacco, alcohol, and human papilloma virus have been shown to be associated with head and neck cancers."
Their prior in vitro and in vivo explorations show the tumorigenic effect of bile at strongly acidic pH on hypopharyngeal cells, enabled through the transcriptional factor NF-κB.
It has also been demonstrated that transcriptional activation of EGFR, STAT3, BCL2, WNT5A, TNF-α, and IL-6, as well as deregulation of “oncomirs” miR-21, miR-155, miR-192 and tumor suppressor miR-375, miR-451a, miR-34a, miR-504 and miR-99a are associated with biliary reflux-related hypopharyngeal squamous cell carcinoma, while in vitro and in vivo explorations have documented that acidic bile-induced oncogenic mRNA and miRNA phenotypes can be prevented by simultaneous co-administration of NF-κB inhibitor in human hypopharyngeal cells.
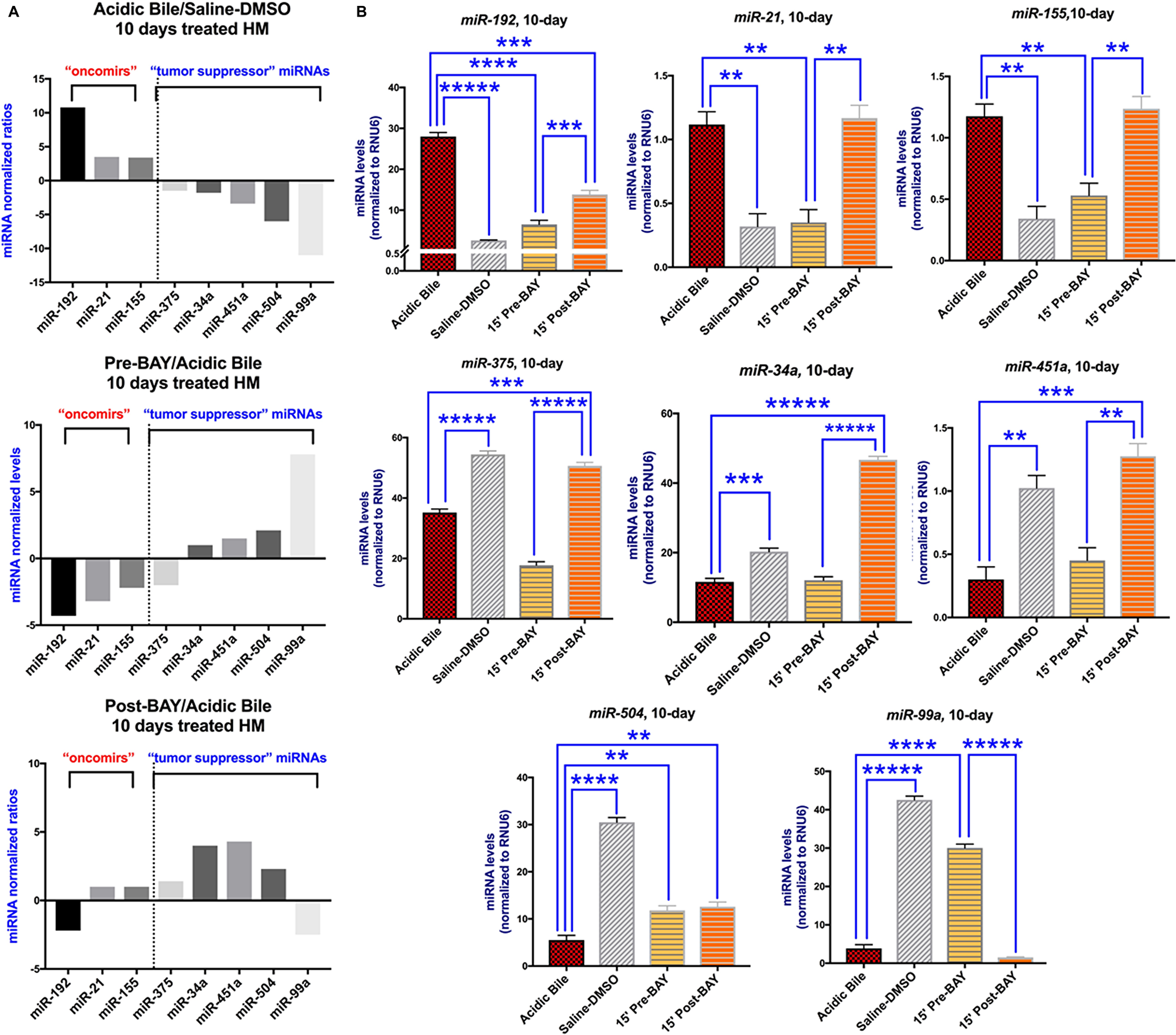
Figure 4: The in vivo pre- or post- topical application of BAY 11-7082 prevents the acidic bile-induced deregulation of cancer-related miRNA markers in 10-day exposed murine HM. (A) Columns of graphs created by Graph Pad Prism software 7.0 depict oncogenic miRNA phenotype (“oncomirs” and “tumor suppressor” miRNAs) in 10-day (two times per day) exposed HM to acidic bile (pH 3.0) vs. saline-DMSO treated HM, pre-BAY 11-7082 + acidic bile (pH 3.0) vs. acidic bile alone treated HM, and acidic bile (pH 3.0) + post-BAY 11-7082 vs. acidic bile alone treated HM (miRNA levels of each target miRNA marker were normalized to RNU6; by real-time qPCR analysis). We observe that pre- or post- application of BAY 11-7082 reverses the acidic bile-induced miRNA phenotype. (B) Graphs, created by Graph Pad Prism 7.0 software, reveal miRNA levels (normalized to RNU6) for each analyzed miRNA marker, comparing HM exposed to acidic bile alone, application of BAY 11-7082 before or after acidic bile and saline-DMSO, by real-time qPCR analysis (p value < 0.05; by t test; multiple comparisons by Holm-Sidak; data obtained from four analyzed samples).
The authors recently showed, using an in vitro model, that a pharmacologic NF-κB inhibitor, BAY 11-7082, applied to hypopharyngeal primary cells, before or after exposure to acidic bile, exerted effects comparable to its simultaneous co-application with acidic bile in successfully inhibiting its cancer-related mRNA and miRNA phenotypes.
To expand their exploration, they hypothesize that in vivo pre- or post- topical exposure of murine hypopharyngeal mucosa to BAY 11-7082, will have similar blocking effects as seen in vitro.
Determining the in vivo temporal characteristics of NF-κB inhibition, using BAY 11-7082, will support future preclinical and clinical trials using NF-κB inhibitors.
"Determining the in vivo temporal characteristics of NF-κB inhibition, using BAY 11-7082, will support future preclinical and clinical trials using NF-κB inhibitors."
The Sasaki Research Team concluded in their Oncotarget Research Paper that based on our previous findings, long-term exposure of the hypopharyngeal mucosa to acidic bile progressively causes its malignant transformation, producing a progressive increase in the positivity of double-strand breaks, oxidative DNA/RNA damage markers, overall p53 and nuclear NF-κB protein levels, as well as the deregulation of NF-κB-related oncogenic mRNA and miRNA phenotypes that are found to precede the histological evidence.
They do not yet know whether inhibition of acidic bile-induced NF-κB, using BAY 11-7082, is capable of directly or indirectly influencing DNA stability in the form of oxidative DNA damage and DSBs, and/or p53 expression, according to previous studies.
Long-term exposure to BAY 11-7082 may further reveal how NF-κB inhibition suppresses the initiation of cancer, which is the subject of active inquiry in their current research program.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27706
Full text - https://www.oncotarget.com/article/27706/text/
Correspondence to - Clarence T. Sasaki - [email protected]
Keywords - NF-κB inhibition, BAY 11-7082, bile, laryngopharyngeal reflux, hypopharyngeal cancer
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



