Oncotarget published "The antitumoral effects of chemerin are independent from leukocyte recruitment and mediated by inhibition of neoangiogenesis" which reported that Chemerin, a multifunctional protein acting through the receptor ChemR23/CMKLR1, is downregulated in various human tumors and was shown to display antitumoral properties in mouse models of cancer. The protective effect of chemerin is mediated by CMKLR1 and appears unrelated to the recruitment of leukocyte populations. The anti-angiogenic effects of chemerin were confirmed in a bead sprouting assay using human umbilical vein endothelial cells.
Dr. Marc Parmentier from The Université Libre de Bruxelles said, "Chemerin is a multifunctional protein of 16 kDa, secreted as an inactive precursor by many cell types, including fibroblasts, myocytes, hepatocytes, adipocytes, and many epithelial cells."
CMKLR1, also named ChemR23 or chemerin1, was the first receptor described for chemerin.
CMKLR1, also named ChemR23 or chemerin1, was the first receptor described for chemerin.
CMKLR1 belongs to the rhodopsin-like family of G protein-coupled receptors and is coupled to the Gi/o family, inhibiting cAMP accumulation and triggering IP3-dependent calcium mobilization and activation of the ERK1/2 and PI3K/AKT cascades. Chemerin also promotes β-arrestin recruitment and efficient CMKLR1 internalization. These proteases include neutrophil elastase, cathepsin G as well as proteases of the coagulation and fibrinolytic cascades.
Bioactive chemerin is therefore generated in inflammatory conditions, following tissue injury and during tissue remodeling. Through the recruitment of leukocyte populations and possibly other mechanisms, chemerin plays both pro-and anti-inflammatory roles in various experimental disease models. Chemerin was reported to contribute to disease states by promoting inflammation in autoimmune encephalomyelitis, chronic obstructive pulmonary disease, and psoriasis, but to dampen inflammation in viral and aseptic lung disease models, atherosclerosis and pancreatitis.
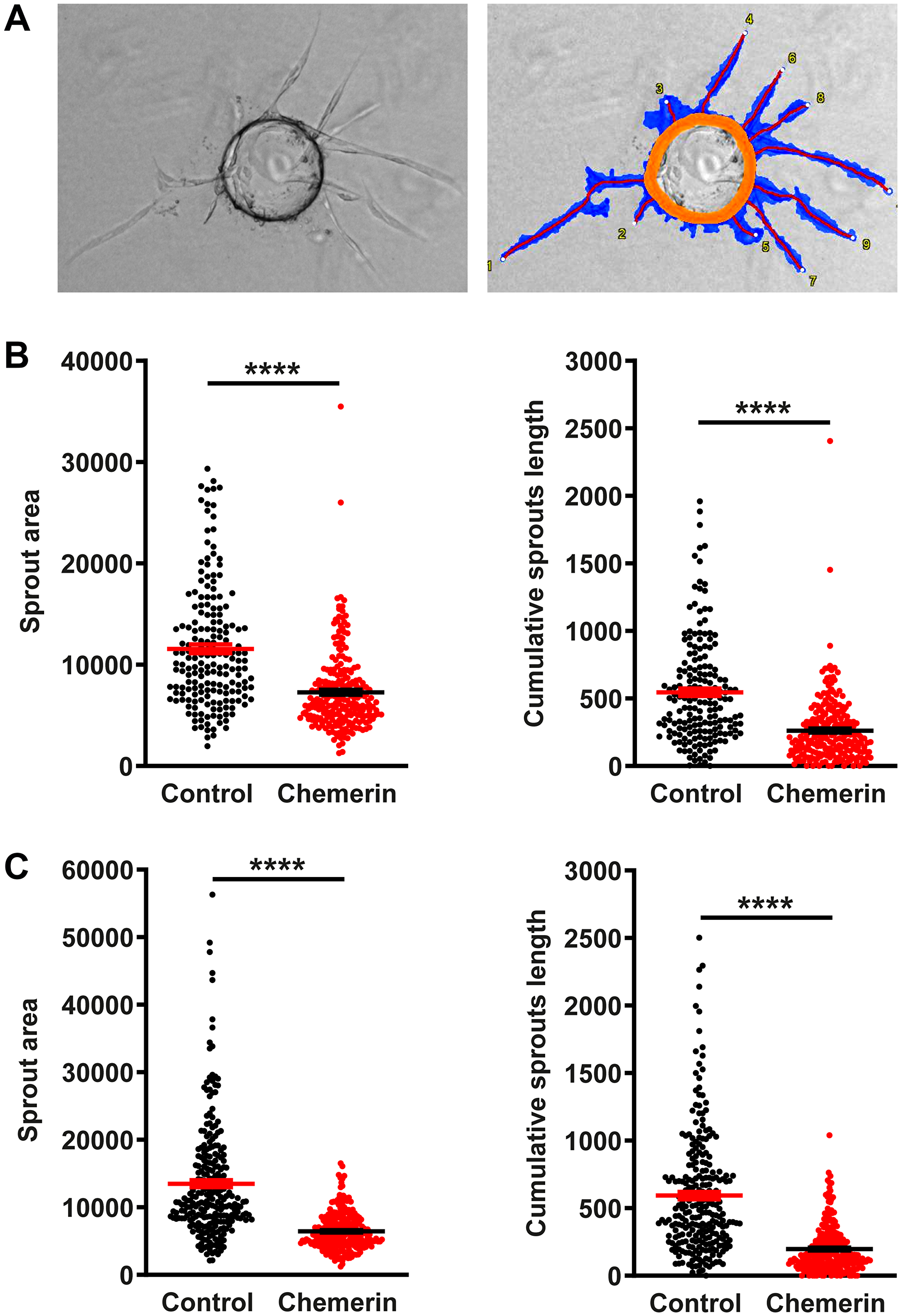
Figure 3: Myo1g over expression is conserved at protein level in acute lymphoblastic leukemia. (A) Intensity of Optical Density (IOD) of Myo1g expression in PBMCs from control individuals n = 61, patients at Diagnostic n = 117, patients at remission n = 105 and patients at consolidation n = 106. Error bars represent mean +/– SD, One-way ANOVA with Dunn's correction, *P = 0 < 0.05. (B) Representative images of Myo1g expression at diagnosis from a patient and a control individual. (C) Mean fluorescence intensity of Myo1g expression in PBMCs from patients with no relapse and with relapse at Diagnostic (D) n = 93, n = 8 respectively, patients at remission n = 79 and n = 4 respectively and patients at consolidation n = 77 and n = 4 respectively. Error bars represent mean +/– SD, Unpaired t test, *P = 0 < 0.05. (D) Representative confocal images of Myo1g expression in 3 patients at diagnostic, remission and consolidation and 3 control individuals, bar represents 10 μm.
Chemerin levels were found elevated in the blood of patients with many different types of cancer. Expression in the tumor itself was most often turned down compared with normal tissue in many cancer types, including breast, lung, and prostate cancers, adrenocortical and hepatocellular carcinomas, melanoma, and squamous cell carcinoma of the skin.
The Parmentier Research Team concluded in their Oncotarget Research Output that, in their K5-chemerin model, bioactive chemerin overexpression does not seem to affect the vasculature in normal tissues, which might lead to severe consequences. Tumoral angiogenesis differs significantly from the normal process. Such a specific effect on tumoral angiogenesis might constitute an advantage in terms of therapeutic applications. Future will tell whether targeting the chemerin-CMKLR1 system with agonists, thereby affecting the formation and/or stabilization of neovessels, may constitute an additional and complementary approach to combat solid tumors in patients.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28056
Full text - https://www.oncotarget.com/article/28056/text/
Correspondence to - Marc Parmentier - [email protected]
Keywords - chemerin, ChemR23, CMKLR1, Rarres2, tumor angiogenesis
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



