Oncotarget recently published "Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer" which reported that the authors investigated whether Trop-2-expression and homologous recombination repair of SN-38-mediated double-strand DNA breaks play a role in the sensitivity of triple-negative breast cancer to SG.
Further, two Trop-2-transfectants of MDA-MB-231, C13, and C39, were treated in mice with SG to determine whether increasing Trop-2 expression improves SG efficacy.
SG mediated >2-fold increase in Rad51 in MDA-MB-231 but had no effect in SK-MES-1 or HCC1806, resulting in lower levels of dsDNA breaks in MDA-MB-231. SG and saline produced similar effects in parental MDA-MB-231 tumor-bearing mice.
However, in mice bearing higher Trop-2-expressing C13 and C39 tumors after Trop-2 transfection, SG provided a significant survival benefit, even compared to irinotecan.
These results suggest that SG could provide better clinical benefit than irinotecan in patients with HRR-proficient tumors expressing high levels of Trop-2, as well as to patients with HRR-deficient tumors expressing low/moderate levels of Trop-2.
SG could provide better clinical benefit than irinotecan in patients with HRR-proficient tumors expressing high levels of Trop-2, as well as to patients with HRR-deficient tumors expressing low/moderate levels of Trop-2
Dr. Thomas M. Cardillo from Immunomedics, Inc said, "In recent years, there has been an increased focus on personalized cancer therapy."
SG demonstrated significant clinical benefit across a range of solid tumors, including metastatic TNBC, hormone-positive breast cancer, small-cell lung cancer, non-small-cell lung cancer, and metastatic urothelial carcinomas.
It remains unclear from these results whether the overriding mechanism for SG sensitivity in these various tumor models is Trop-2 expression or defective HRR pathways, or their combination.
Herein, the Oncotarget authors examined the HRR response in MDA-MB-231, being unresponsive to SG, including upregulation of Rad51 and levels of dsDNA breaks mediated by SG exposure, and compared it to that of SG-sensitive tumor lines to elucidate the role that this pathway plays in protecting cells from SG-mediated dsDNA breaks.
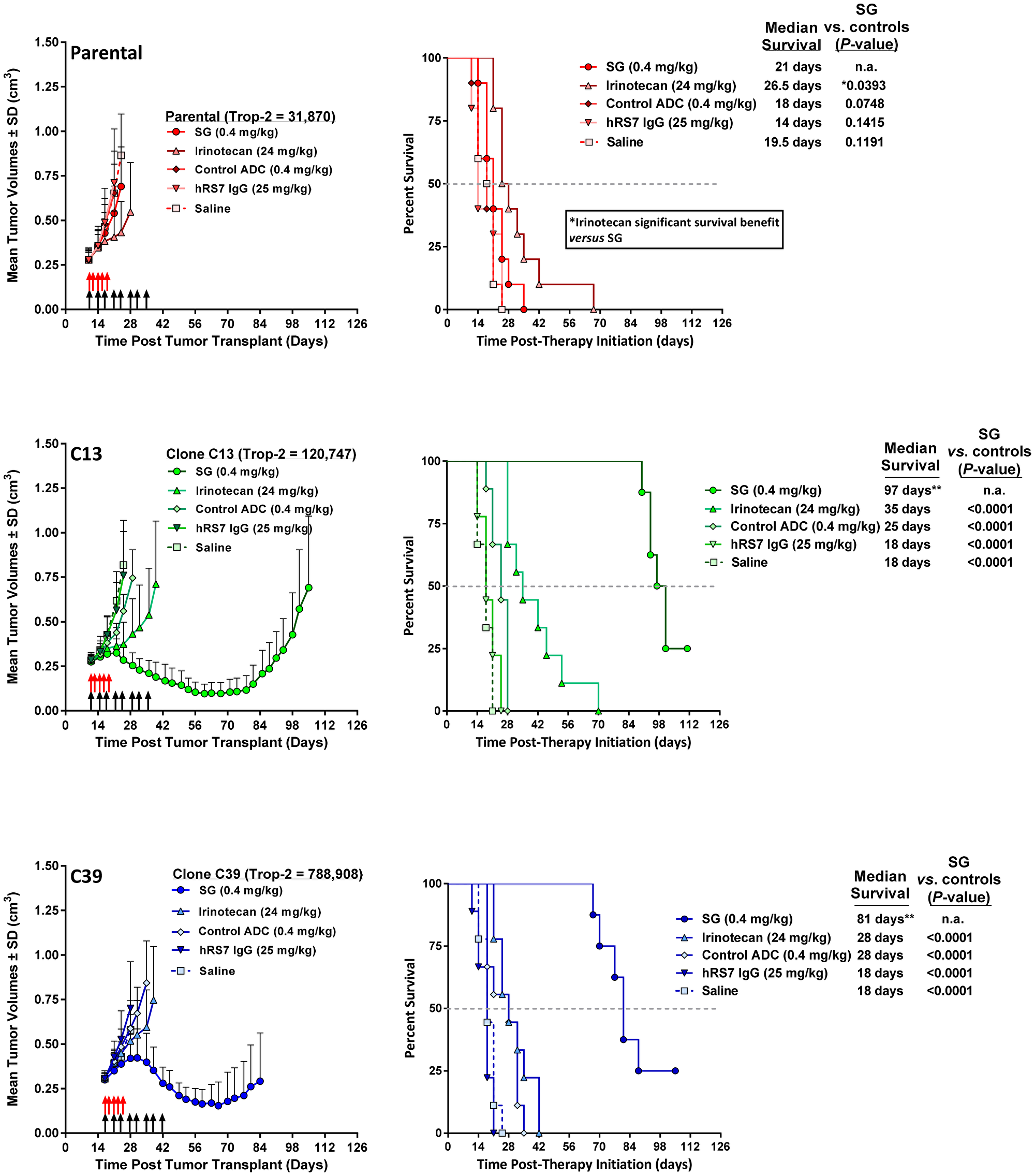
Figure 4: Increased Trop-2 expression in MDA-MB-231 tumors overcomes resistance to SG but not irinotecan. NCr athymic nu/nu mice were injected s.c. with either MDA-MB-231 parental cells (parental), MDA-MB-231 clone 13 (C13) cells, or MDA-MB-231 clone 39 (C39) cells as described in Materials and Methods. Once tumors reached ~0.3 cm3 in size, mice were randomized into the various treatment groups. SG, control ADC, and parental hRS7 IgG antibody, were administered i.p., twice weekly for 4 weeks (black arrows). Irinotecan was administered i.v. at its MTD (q2dx5; red arrows). For all animal studies, the doses of SN-38 immunoconjugates and irinotecan are shown in SN-38 equivalents. The dose of hRS7 is shown at its protein dose equivalent to SG protein dose. Graphs to the left show mean tumor growth curves for each treatment group while those on the left indicate survival curves for these same groups of animals. **One mouse in SG group deemed an outlier via Grubbs' test and removed from final analysis. Grey dotted line in survival curves indicates 50% survival line.
Additionally, MDA-MB-231 cells transfected to express higher levels of Trop-2 were assessed in vivo for SG antitumor effects in comparison to parental tumors with low Trop-2 expression.
However, this does not rule out SG being active in tumors with low Trop-2 expression and deficiencies in HRR.
The Cardillo Research Team concluded in their >Oncotarget Research Paper that, these data strongly support the hypothesis that as a biomarker, high surface Trop-2 expression on a patient's tumor may be predictive of a positive clinical outcome for SG therapy.
Further, there are secondary biomarkers that may need to be considered for those patients with low/moderate Trop-2 expression or those with high Trop-2 expression that failed previous irinotecan therapy for reasons other than acquired resistance.
Moreover, while high expression of Trop-2 was found to be a primary biomarker for SG efficacy, it should not be a limiting factor, because other secondary biomarkers coupled with Trop-2 expression may likewise be predictive of clinical benefit.
For these reasons, future clinical trials will need to comprehensively examine potential biomarkers, in addition to Trop-2 expression, to generate a profile that will better identify those patients likely to benefit from SG therapy.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27766
Full text - https://www.oncotarget.com/article/27766/text/
Correspondence to - Thomas M. Cardillo - [email protected]
Keywords - sacituzumab govitecan, Trop-2, biomarker, RAD51, triple-negative breast cancer
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105




