Volume 11, Issue 19 of @Oncotarget reported that a key requirement for the formation of this oncogenic protein-protein interaction is the conformational flexibility of LMO2.
Here the authors identify a small molecule inhibitor of the SCL-LMO2 PPI, which hinders the interaction in vitro through direct binding to LMO2.
Dr. Erika J. Mancini from the School of Life Sciences, Biochemistry Department, University of Sussex, Falmer, Brighton, BN1 9QG, United Kingdom said, "Stem cell leukemia (SCL, also known as TAL-1) is a basic helix-loop-helix transcription factor with essential, non-redundant roles in haematopoietic development and the terminal maturation of erythroid cell lineages."
"Stem cell leukemia (SCL, also known as TAL-1) is a basic helix-loop-helix transcription factor with essential, non-redundant roles in haematopoietic development and the terminal maturation of erythroid cell lineages."
- Dr. Erika J. Mancini, School of Life Sciences, Biochemistry Department, University of Sussex
Ectopic expression of SCL through chromosomal translocations or microdeletion or transactivation through an abnormal expression of proteins within the haematopoietic regulatory network is observed in ~60% of T-ALL cases of which ~40% also display abnormal LMO2 expression.
Studies of the pre-leukemic phenotype in mouse models suggest that both SCL and LMO2 overexpression causes T-ALL by inducing aberrant self-renewal of committed T-cells in the thymus, resulting in a cellular pool which can over time acquire additional gain-of-function mutations of genes involved in signaling pathways regulating T-cell development, such as NOTCH1, interleukin-7, KIT and FLT3.
Numerous studies have suggested that SCL and LMO2 function cooperatively to drive this phenotype of aberrant self-renewal in T-cell precursors through a mechanism of functional sequestration of E2A-protein homodimers which are essential for normal T-cell differentiation.
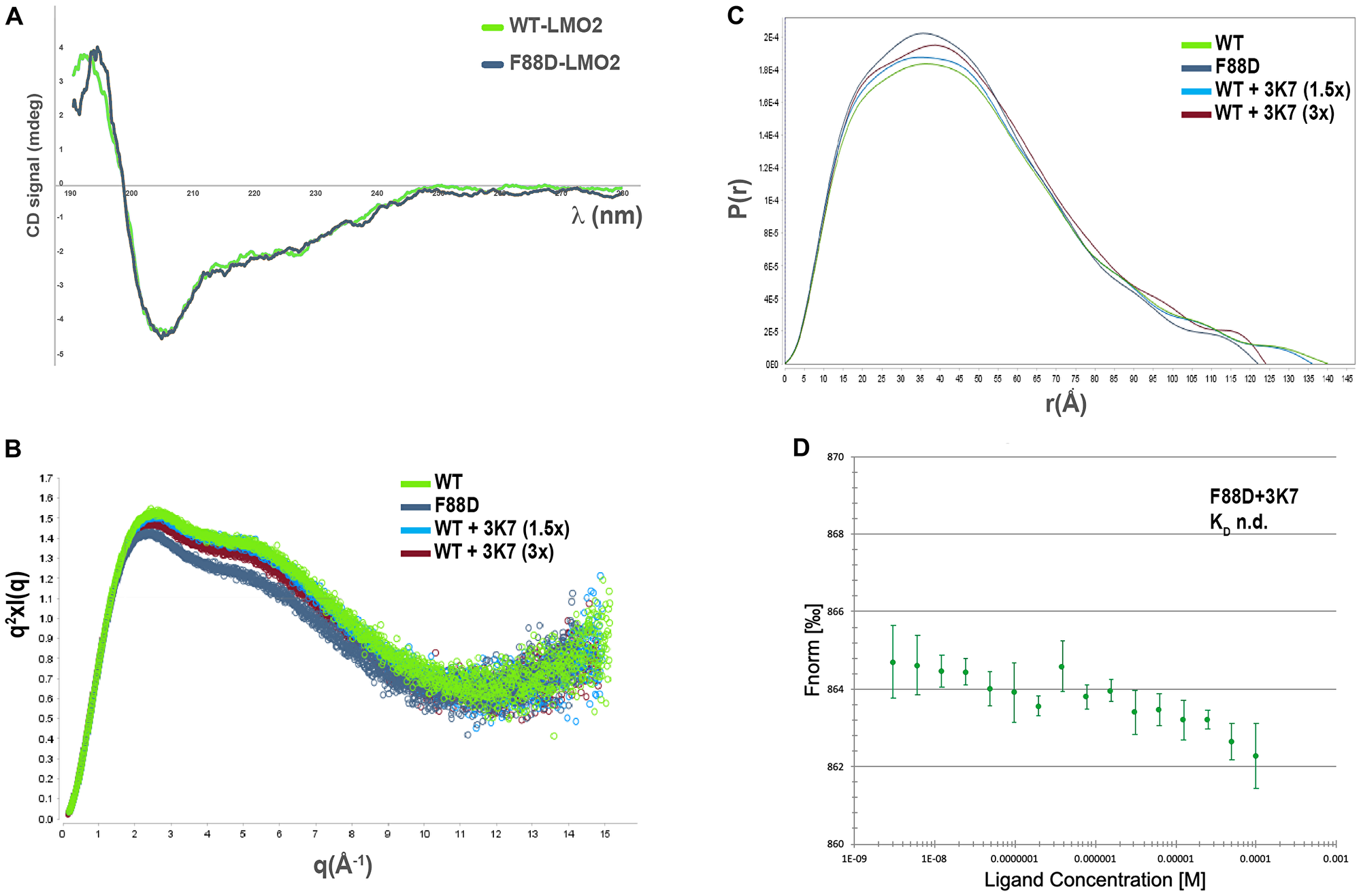
Figure 5: 3K7 induces a change in LMO2 conformation comparable to LMO2-F88D. (A) Comparison of the far-UV CD spectra for LMO2 (green) and F88D (gray) shows profiles consistent with folded proteins containing similar secondary structures elements. (B) Kratky plot of the solution scattering showing broad bell-shaped curves typical of elongated, flexible protein molecules (green: LMO2; gray: LMO2-F88D; light blue: LMO2+ 1.5x 3K7; red: LMO2+3x 3K7). (C) Overlay of the P(r) distribution curves characterizing LMO2 (green), LMO2-F88D (gray), and LMO2 incubated with 1.5× (light blue) and 3× (red) molar concentrations of 3K7. The goodness of the data fitting was assessed by calculation of the χ2 value, with best fit approximating to 1. The shape of curves is characteristic for elongated molecules. (D) Curve showing normalised fluorescence data from MST experiments with LMO2-F88D with increasing 3K7 concentrations. Error bars represent standard deviation, n = 3.
As the binding affinity of LMO2 for SCL/E2A-containing complexes is very high, the sequestration of E2A proteins is strongly favored in cells that express both SCL and LMO2.
Their work suggests that developing small molecules interfering with the conformational flexibility of LMO2 is a realistic and novel approach for the therapeutic targeting of T-ALL.
The Mancini Research Team concluded in their Oncotarget Research Paper, "The low aqueous solubility of 3K7 prevented additional cellular assays to be performed, restricting the prospect of testing this compound in vivo. Low solubility also prevented a more in-depth structural analysis of the binding mode of 3K7, limiting further chemical development of the compound. Nonetheless, our data provide evidence that the effect of small molecules on modulating protein conformation can be potentially used as a powerful approach to drug discovery, even for otherwise intractable PPIs. Specifically, as protein conformation is key to mediating PPIs between flexible, minimally structured proteins, future work could exploit this reliance on conformational flexibility to engineer specific therapeutics that target transcription factors.
However, we posit that not all PPIs are equally tractable for blocking by small molecules that modulate conformational flexibility and detailed analysis of PPI interfaces is critical for the selection of those with the highest chance of success."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27580
Full text - https://www.oncotarget.com/article/27580/text/
Correspondence to - Erika J. Mancini - [email protected]
Keywords - protein-protein interaction, leukaemia, T-ALL, drug discovery, LMO2
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


