Volume 11, Issue 23 of @Oncotarget reported that the authors have previously established that mi R-151a functions as an onco-mi R in non-small cell lung cancer cells by inducing partial EMT and enhancing tumor growth.
Here, the authors identify anti-mi R-151a as a molecule that promotes endothelial cell contacts and barrier properties, suggesting that mi R-151a regulates cell-cell junctions.
They find that induced mi R-151a expression enhances endothelial cell motility and angiogenesis and these functions depend on mi R-151a-induced Slug levels.
Moreover, The authors show that mi R-151a overexpression enhances tumor-associated angiogenesis in 3D vascularized tumor spheroid assays.
Their results suggest that mi R-151a plays multi-faceted roles in the lung, by regulating multiple functions in distinct cell types.
Dr. Irene Munk Pedersen from The University of California as well as The Scintillon Institute said, "Angiogenesis, or the growth of new networks of blood vessels from existing vessels, is an important natural process used for growth, healing, and reproduction."
"The authors find that anti-mi R-375 and anti-mi R-151a strengthen cell-cell contact and endothelial cell barrier in primary lung endothelial cells, relative to control samples"
Proper regulation of angiogenesis is essential not only for developing an adult's healthy organs to support growth and metabolism but also for disease progression since abnormal vessel growth and/or function are hallmarks of cancer, ischemic and chronic inflammatory diseases.
Slug and Snail may regulate a distinct but overlapping set of genes and therefore Snail may compensate for Slug in the Slug-knock-out context.
There is a need to characterize the repertoire of master mi R regulators in normal and diseased microenvironments to further understand how to restore the delicate balance of cell homeostasis when it has been lost.
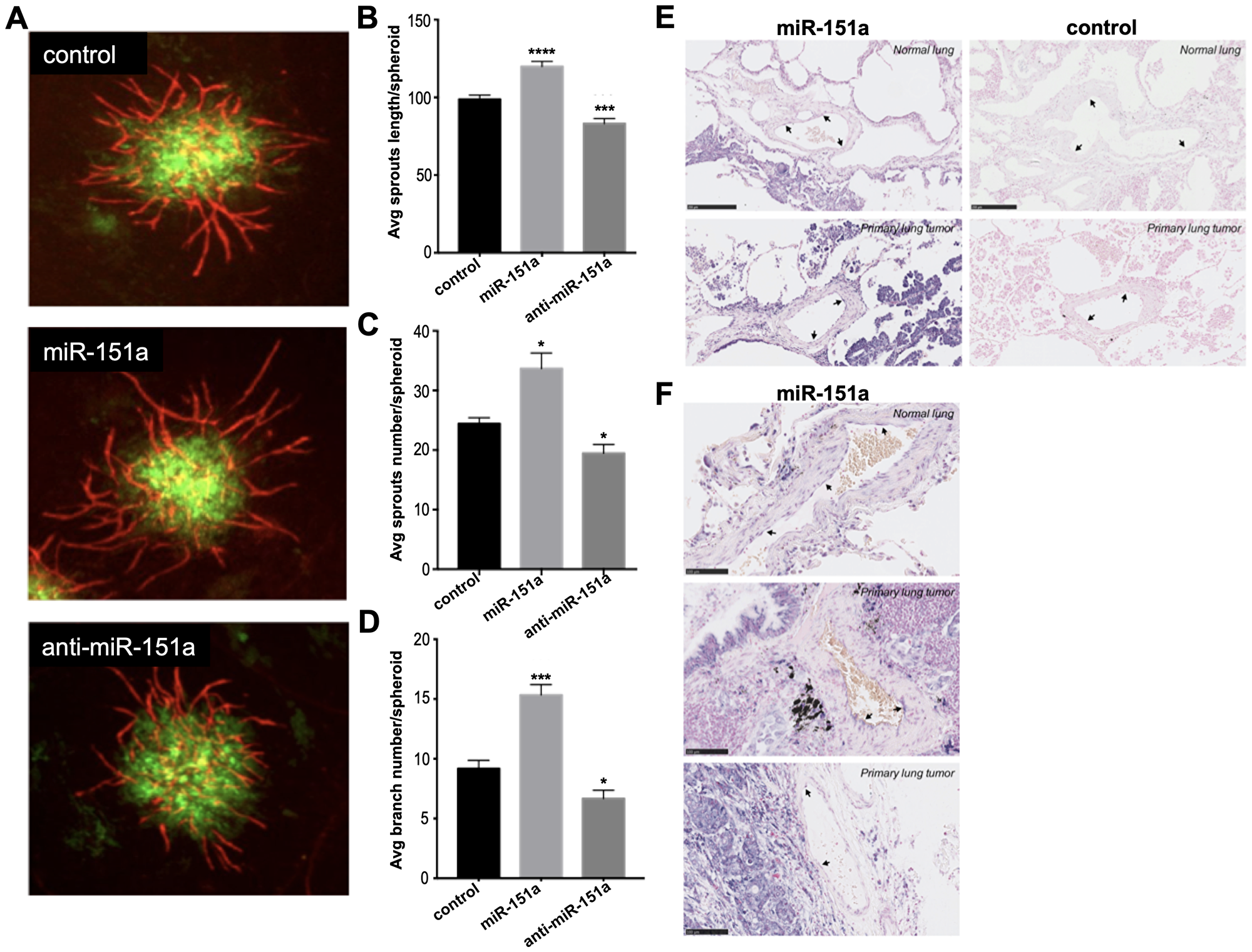
Figure 5: miR-151a enhances angiogenesis in 3D vascularized lung tumor spheroids and is expressed in vasculature of NSCLC patient specimens. (A) Stable miR-modulated (GFP-expressing miR control, miR-151a and anti-miR-151a) HUVEC and lung cancer cells (A549) were generated. miR-modulated lung cancer and endothelial cells were mixed and 3D vascularized tumor spheroids were allowed to form. On day 7 tissues were fixed and immunofluorescent staining was performed visualizing vessels (CD31, red) and tumor cells (GFP, green). Representative images from one of at least three similar experiments are shown. Scale bars: 150 μm. Average (Avg) sprout length per spheroid (B) average sprout number per spheroid (C) and average branch number per spheroid (D) are shown. (E and F) The expression level of miR-151a was analyzed by in situ hybridization in two normal lung samples and three primary NSCLCs (miR-151a expression: high = purple, low = light pink). Scale = 50 μm in Figure 5E and scale = 100 μm in Figure 5F.
The authors find that anti-mi R-375 and anti-mi R-151a strengthen cell-cell contact and endothelial cell barrier in primary lung endothelial cells, relative to control samples.
The Munk Pedersen Research Team concluded in their Oncotarget Priority Research Paper, "Our results show that increased miR-151 expression, significantly promotes endothelial cell motility and angiogenesis in 2D and 3D models and that miR-151a-induced Slug expression is required for these endothelial cell properties. Our findings provide a new avenue to the understanding of the processes in the lung niche environment, and may facilitate the development of potential therapeutics against lung cancer."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27331
Full text - https://www.oncotarget.com/article/27331/text/
Correspondence to - Irene Munk Pedersen - [email protected]
Keywords - angiogenesis, miR-151a, Slug, tumor microenvironment, endothelial cell
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


