Oncotarget published "Ibuprofen disrupts a WNK1/GSK3β/SRPK1 protein complex required for expression of tumor-related splicing variant RAC1B in colorectal cells" which reported that although the molecular mechanism behind the antitumor properties of NSAIDs has been largely attributed to inhibition of cyclooxygenases , several studies have shown that the chemopreventive properties of ibuprofen also involve multiple COX-independent effects.
One example is its ability to inhibit the alternative splicing event generating RAC1B, which is overexpressed in a specific subset of BRAF-mutated colorectal tumors and sustains cell survival.
Here the authors describe the mechanism by which ibuprofen prevents RAC1B alternative splicing in a BRAF mutant CRC cell line: it leads to decreased translocation of SRPK1 and SRSF1 to the nucleus and is regulated by a WNK1/GSK3β/SRPK1 protein kinase complex.
Surprisingly, they demonstrate that ibuprofen does not inhibit the activity of any of the involved kinases but rather promotes disassembly of this regulatory complex, exposing GSK3β serine 9 to inhibitory phosphorylation, namely by AKT, which results in nuclear exclusion of SRPK1 and SRSF1 hypophosphorylation.
The Oncotarget data shed new light on the biochemical mechanisms behind ibuprofen's action on alternative spliced RAC1B and may support its use in personalized approaches to CRC therapy or chemoprevention regimens.
The Oncotarget data shed new light on the biochemical mechanisms behind ibuprofen's action on alternative spliced RAC1B and may support its use in personalized approaches to CRC therapy or chemoprevention regimens.
Dr. Peter Jordan from The National Health Institute Dr. Ricardo Jorge as well as The University of Lisbon said, "Cancer is the second leading cause of death globally [1] and one major risk factor for tumor development is chronic inflammation."
A long term use of nonsteroidal anti-inflammatory drugs, like ibuprofen and aspirin, which are among the most commonly prescribed medications worldwide, was shown to provide chemoprevention against various types of cancer.
Ibuprofen, like most NSAIDs, inhibits both COX isoforms so that side-effects such as intestinal bleeding or cardiovascular disease can occur, questioning the long-term use of NSAIDs for cancer chemoprevention.
Interestingly, some NSAIDs were reported to inhibit tumor growth by targeting other cellular processes and elucidation of the underlying biochemical processes could lead to the development of safer and more efficacious drugs for cancer chemoprevention or adjuvant therapy.
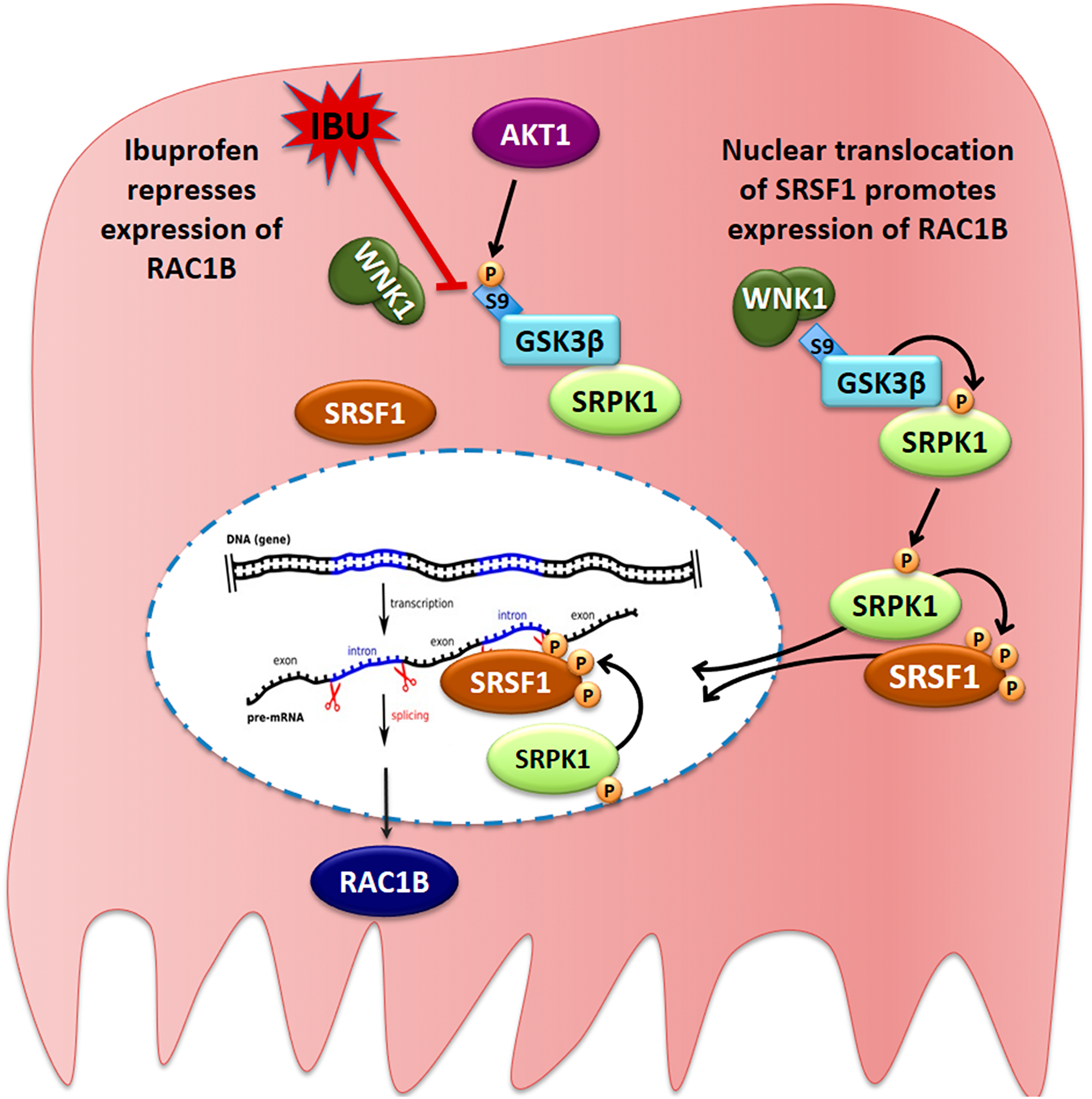
Figure 9: Proposed model for the ibuprofen-inhibited expression of alternative spliced RAC1B. HT29 cells represent a subgroup of colorectal cancer cells, in which alternative splicing of the RAC1 pre-mRNA yields a second transcript that becomes translated into the tumor-promoting RAC1B protein variant. In these cells, a cytoplasmic protein complex composed of WNK1, GSK3β and SRPK1 assures that GSK3β can phosphorylate SRPK1, allowing its translocation into the nucleus. SRPK1 phosphorylates the splicing factor SRSF1, first in the cytoplasm to promote its nuclear translocation, and then inside the nucleus to retain SRSF1 so that it can bind to its specific recognition motif in exon 3b of the RAC1 pre-mRNA. This promotes exon 3b inclusion and subsequent expression of alternative spliced RAC1B. In the presence of ibuprofen or after experimental depletion of endogenous WNK1, the interaction between WNK1 and GSK3β is disrupted, leading to exposure of the N-terminal serine 9 of GSK3β, which is then phosphorylated by AKT1. This phosphorylation is inhibitory and prevents GSK3β from phosphorylating SRPK1. As a result, SRPK1 remains cytosolic, nuclear phosphorylation of SRSF1 is no longer sustained, and SRSF1 leaves the nucleus so that exon 3b is no longer recognized by the spliceosome.
In the case of ibuprofen, numerous studies have shown that its cancer chemopreventive properties are much more complex and involve multiple COX-independent effects.
The authors show that ibuprofen disrupts a signal transduction pathway by, unexpectedly, interfering with the assembly of a protein kinase complex, composed by WNK1, GSK3β and SRPK1. This leads to changes in the subcellular localization of splicing factor SRSF1, which promotes inclusion of exon 3b into the mRNA and subsequent expression of RAC1B.
The Jordan Research Team concluded in their Oncotarget Research Output, "our data suggest that ibuprofen treatment interferes with a signal transduction pathway involved in the regulation of alternative spliced RAC1B. The proposed model is schematically depicted in Figure 9. One other report on prostate cancer cells receiving combined treatment of ibuprofen and epigallocatechin-3-gallate, reported changes in alternative splicing, in particular promoting the shorter and proapoptotic BCL-X (S) or MCL-1(S) variants [43]."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27816
Full text - https://www.oncotarget.com/article/27816/text/
Correspondence to - Peter Jordan - [email protected]
Keywords - ibuprofen, protein kinase, RAC1B, alternative splicing, colorectal cancer cells
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



