Oncotarget published "High-fat diet-fed ovariectomized mice are susceptible to accelerated subcutaneous tumor growth potentially through adipose tissue inflammation, local insulin-like growth factor release, and tumor associated macrophages" which reported that the association between obesity and colorectal cancer (CRC) risk has been well established. This relationship appears to be more significant in men than in women, which may be attributable to sex hormones - controlled animal studies to substantiate these claims and the mechanisms involved are lacking. MC38 murine colon adenocarcinoma cells were injected subcutaneously into high-fat diet fed male, female and ovariectomized female C57BL/6 mice.
HFD OVX mice exhibited the most significant tumor growth compared to HFD male and female mice and this was associated with increased subcutaneous adipose tissue.
Further, the subcutaneous adipose tissue depots within HFD OVX mice exhibited more severe macrophage associated inflammation compared to female, but not male mice.
Conditioned media from subcutaneous adipose tissue of HFD OVX contained higher IGF-1 levels compared to male, but not female mice.
Finally, HFD OVX mice had increased M2-like gene expression in their tumor-associated macrophages compared to female mice.
Dr. Angela Murphy from The University of South Carolina said, "There is convincing evidence that excess body weight is associated with increased risk for late onset (> 50 years of age) colorectal cancer (CRC)."
There is convincing evidence that excess body weight is associated with increased risk for late onset (> 50 years of age) colorectal cancer (CRC)
Taken together, the current literature suggests that:
- sex disparities are evident in obesity-enhanced CRC, and
- hormonal status likely plays a role in CRC risk in women
These perturbations have been associated with CRC risk and are likely biological factors that link obesity to CRC risk.
For instance, the homeostasis model of risk assessment-insulin resistance has been reported to be associated with risk for CRC.
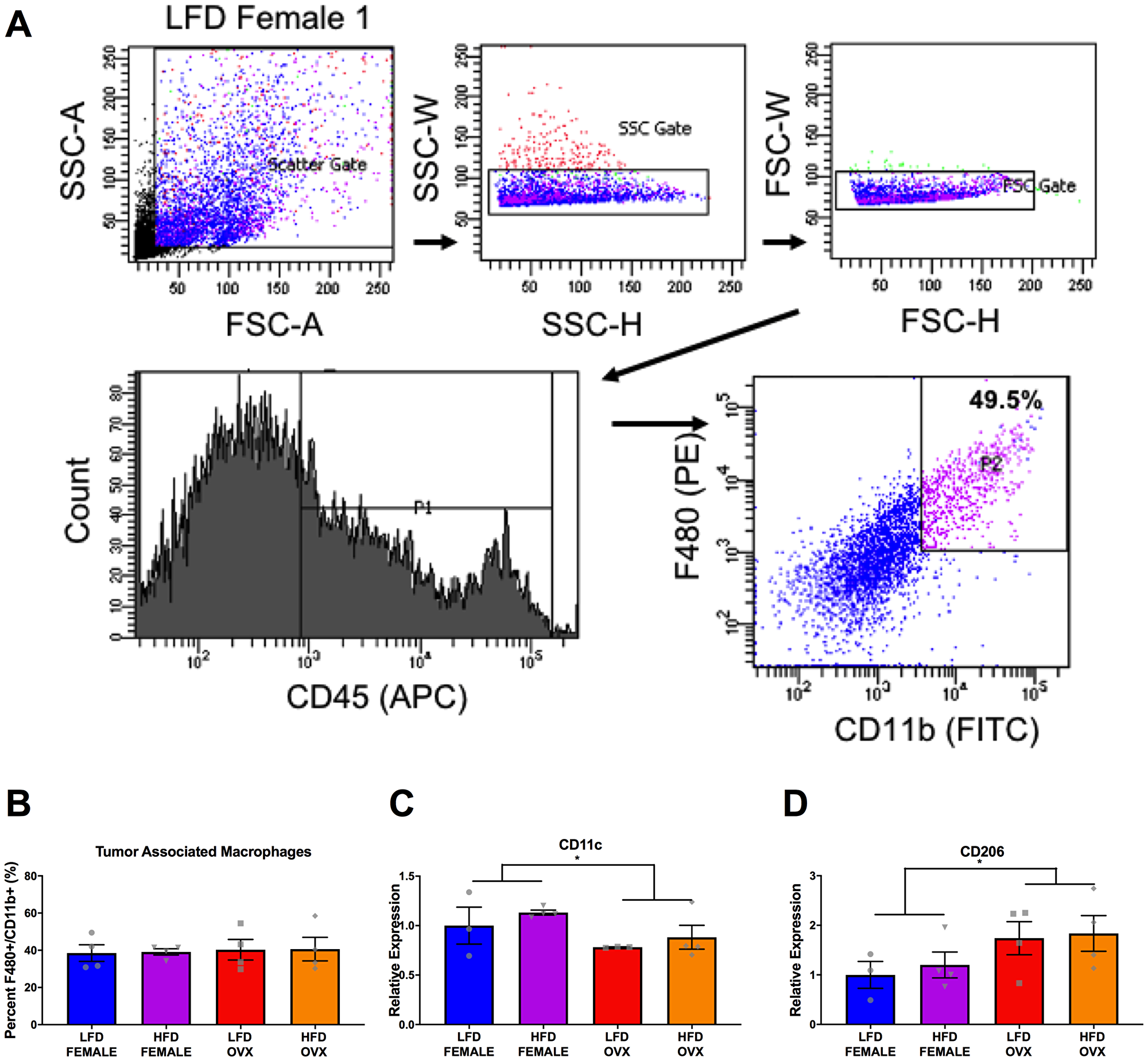
Figure 6: Differential tumor-associated macrophage phenotype in female and OVX mice. (A) Representative gating strategy for sorting of tumor associated macrophages, defined as CD45+ F4/80+ CD11b+ cells. (B) Percentage of tumor associated macrophages within tumors at the time of sort. (C and D) Relative gene expression of CD11c (C) and CD206 (D) in collected tumor associated macrophages. Ct values relative to average of multiple internal controls determined using qBASE pro software analysis. Ct values normalized to LFD Female group. Values are means ± SE; n = 3–4 mice per group. Bar graphs not sharing a common letter are significantly different from one another (p ≤ 0.05).
As hormone status is an influential risk factor for both obesity and CRC, the Oncotarget authors also sought to examine the effect of ovarian hormone deficiency on obesity-enhanced CRC.
Overall, this study provides insight into the contributions of hormonal status, fat distribution, and local versus systemic effects of obesity on CRC risk.
The Murphy Research Team concluded in their Oncotarget Research Paper, "our study confirms that established obesity following 20–21 weeks of high-fat-diet feedings enhanced the growth of subcutaneously implanted MC38 tumors, but this appears to be largely due to the increase in tumorigenesis in the OVX mice. We established that diet induced obesity and associated insulin resistance manifest differently in male, female, and OVX mice. Although we were unable to completely replicate epidemiological data, we report that obese OVX mice are most vulnerable to accelerated subcutaneous tumor growth in the MC38 model. Our data suggest several potential mechanisms including increased local adiposity, increased local inflammation and IGF-1, and polarization of macrophages to an M2 phenotype, providing insight into this obesity enhanced tumor growth in OVX mice."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27832
Full text - https://www.oncotarget.com/article/27832/text/
Correspondence to - Angela Murphy - [email protected]
Keywords - colorectal cancer, obesity, metabolism, macrophage, inflammation
About Oncotarget
Oncotarget is a weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



