Oncotarget published "High CD39 expression is associated with the non-muscle-invasive phenotype of human bladder cancer" which reported that each miRNA can regulate the expression of more than several hundred genes by binding to the 3′UTR untranslated region of their target based on complementary sequences, blocking translation or causing the degradation of target mRNAs.
Papillary thyroid cancer corresponds to approximately 85% of malignant thyroid cancers. Until a few years ago, PTC was characterized as a cancer presenting essentially upregulated miRNAs although in multiple human cancers a general downregulation of miRNAs is generally observed. MiR-7-5p can arise from three different loci and is highly conserved, supporting its key role.
Dr. Janaina Mendes Ferreira from Universidade Nove de Julho said, "Bladder cancer (BC) is the most common malignancy of the urinary tract, being the fourth most common cancer in men and the ninth in women."
Approximately 70% to 80% of BC cases are non-muscle-invasive, but a significant portion of those patients recurs and progresses to muscle-invasive form despite transurethral resection and adjuvant intravesical therapies. One of these enzymes is the CD39, an integral membrane protein that hydrolyzes ATP and ADP in order to yield AMP in the extracellular space. The CD39 enzyme usually works with another enzyme named CD73, which dephosphorylates AMP into adenosine.
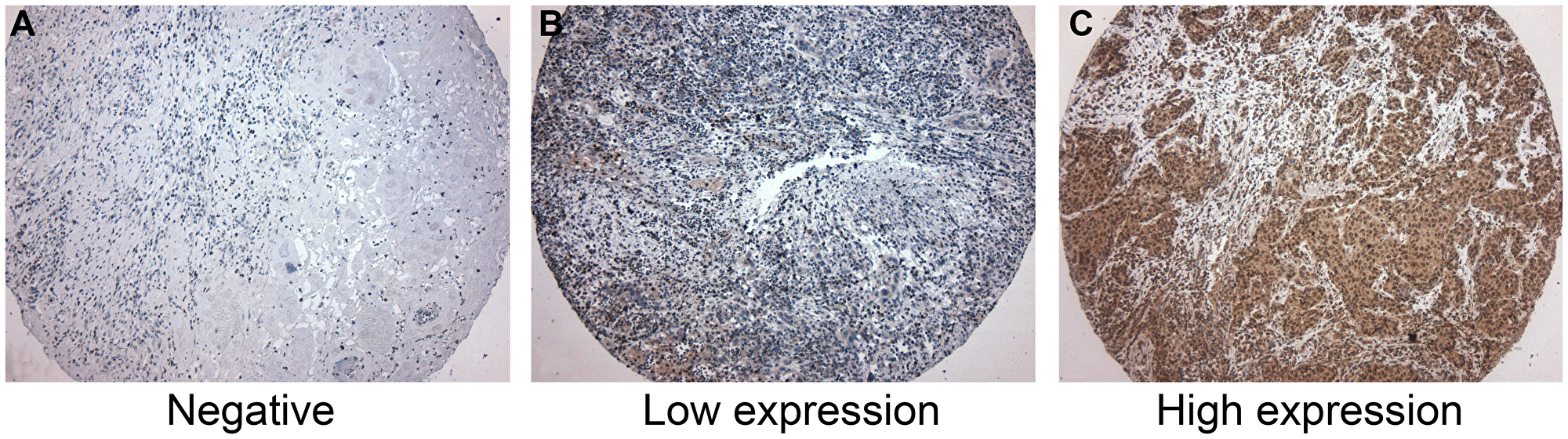
Figure 1: Immunohistochemistry for CD39. (A) Negative for CD39 expression. (B) Low CD39 expression. Few neoplastic cells were positive for CD39. (C) High CD39 expression, showing more than 50% of positive cells for CD39. CD39 expression was predominantly cytoplasmatic in neoplastic cells. CD39-expressing inflammatory cells were not considered in this study.
There is a distinct pattern in metabolizing nucleotides between NMI and MI bladder tumors. Working with human BC cell lines they demonstrated that BC cells with low malignancy phenotype established from a non-muscle invasive tumor exhibited a high level of hydrolysis of tri- and diphosphonucleosides due to high expression of NTPDase 3. In contrast, cells with a high malignancy phenotype derived from a muscle-invasive tumor exhibited a robust reduction in the NTPDase 3 expression. A few years later the same group worked with a chemical-induced bladder tumor model demonstrating that the immunostaining for NTPDase is progressively lost during the disease progression, in contrast to CD73 expression that increased over time.
Considering the exposed above, these authors hypothesized that information on the expression of CD39 and CD73 in human specimens of bladder tumor could be useful in the prognosis of BC. In the present study, they investigated the proteic expression of CD39 and CD73 in patients who underwent surgery for the treatment of NMI and MI urothelial bladder carcinoma.
They investigated the proteic expression of CD39 and CD73 in patients who underwent surgery for the treatment of NMI and MI urothelial bladder carcinoma
The Ferreira Research Team concluded in their Oncotarget Research Output, "the altered expression of CD39 presented herein supports the idea that this ectonucleotidase may be involved in bladder tumorigenesis. Our results suggest that malignant urothelial cells of human BC strongly express CD39; however, high expression of CD39 in tumor cells is correlated with the early stage of BC. Further studies are necessary to clarify the effective role of loss/downregulation of CD39 expression in the establishment of invasive phenotype in BC cells."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28029
Full text - https://www.oncotarget.com/article/28029/text/
Correspondence to - Janaina Mendes Ferreira - [email protected]
Keywords - bladder cancer, purinergic signaling, ectonucleotidases, CD39
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



