Oncotarget published "Genomic and neoantigen evolution from primary tumor to first metastases in head and neck squamous cell carcinoma" which reported that prior work has characterized changes in the mutation burden between primary and recurrent tumors; however, little work has characterized the changes in neoantigen evolution.
These authors characterized genomic and neoantigen changes between 23 paired primary and recurrent head and neck squamous cell carcinoma (HNSCC) tumors.
Within these tumors, they identified 6 genes which have predicted neoantigens in 4 or more patients.
Within HNSCC tumors examined in this Oncotarget research paper, there are neoantigens in shared genes by a subset of patients.
The presence of neoantigens in these shared genes may promote an anti-tumor immune response which controls tumor progression.
Dr. Brian A. Van Tine from The Washington University in St. Louis, The St. Louis Children's Hospital as well as The Siteman Cancer Center said, "Head and neck cancer are a group of heterogeneous tumors with an estimated 644,000 new cases per year worldwide."
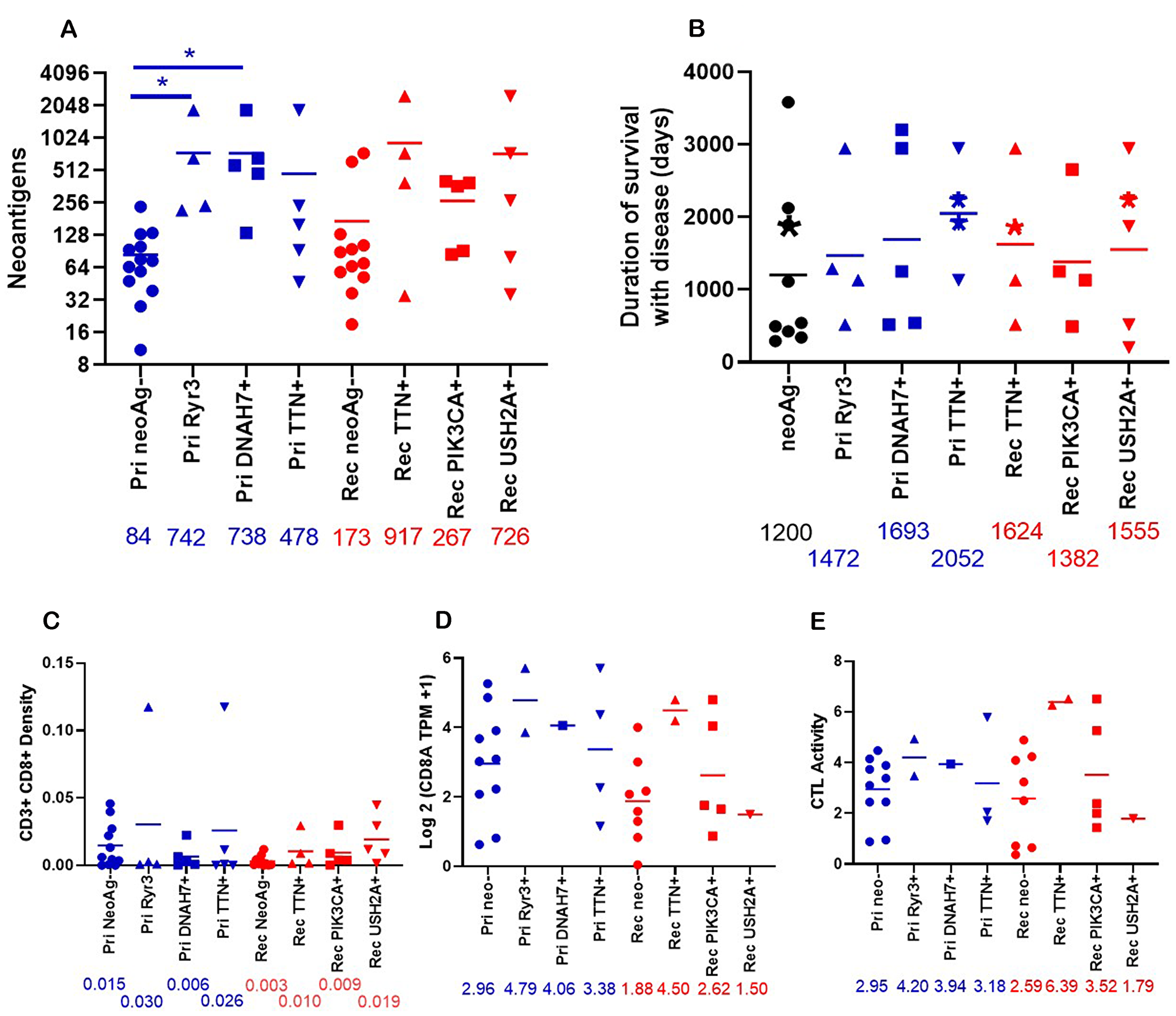
Figure 6: Properties of patients who have neoantigens in shared genes. (A) The total number of neoantigens was graphed for primary tumors with neoantigens in Ryr3 (n = 4), DNAH7 (n = 5), TTN (n = 5), or no neoantigens (Pri neoAg-, (n = 13)) or relapse tumors with neoantigens in TTN (n = 4), PIK3CA (n = 5), USH2A (n = 5), or no neoantigens (Rel neoAg-, (n = 12)). The numbers under the X axis are the mean of neoantigens. *indicates p < 0.05. (B) The duration of disease for patients with predicted neoantigens in the Primary tumor (Ryr3 (n = 4), DNAH7 (n = 5), TTN (n = 4)) and relapse tumor (TTN (n = 4), PIK3CA (n = 4), and USH2A (n = 5)), or no predicted neoantigens in these genes (neoAg-, (n = 9)). Patients with neoantigens in multiple genes are placed in all neoantigens. The asterisks indicate patients who were alive as of writing. (C) The density of CD3+ CD8+ cells in the tumor graphed by presence of neoantigens in shared genes. Primary RYR3 (n = 4), Primary DNAH7 (n = 5), Primary TTN (n = 5), Primary no neoantigens (n = 11), relapse TTN (n = 4), relapse PIK3CA (n = 5), relapse USH2A (n = 5) relapse no neoantigens (n = 10)). Numbers under the X axis are the mean of the density. (D) The Log2 (TPM +1) expression of CD8A was graphed by the presence of neoantigens in shared genes. Pri NeoAg- (n = 10), Pri Ryr3+ (n = 2), Pri DNAH7+ (n = 1), Pri TTN+ (n = 4), Rel neoAg- (n = 8), Rel TTN+ (n = 2), Rel PIK3CA+ (n = 5), Rel USH2A+ (n = 1). The number under the X axis is the mean for each column. (E) The CTL activity was graphed by neoantigen status. CTL activity was calculated as described in Figure 4. Pri NeoAg- (n = 10), Pri Ryr3+ (n = 2), Pri DNAH7+ (n = 1), Pri TTN+ (n = 3), Rel neoAg- (n = 8), Rel TTN+ (n = 2), Rel PIK3CA+ (n = 5), Rel USH2A+ (n = 1). The number under the X axis is the mean for each column.
The infiltration of immune cells, including T cells, into tumors is associated with improved outcomes and longer survival in HNSCC.
The infiltrating T cells release granules containing perforin and granzyme A and B which directly kill tumor cells or release other cytokines and chemokines that promote the anti-tumor immune response and alter the tumor microenvironment.
For example, infiltrating T cells release interferon gamma which increases expression of PD-L1 and CTLA-4, which may increase the efficacy of immune checkpoint therapy.
Multiple studies have characterized changes in mutation burden in HNSCC, when comparing primary and metastatic tumors, no studies have characterized the shifting neoantigen burden between primary and metastatic tumors within HNSCC.
In this Oncotarget study, the authors characterized the mutational and neoantigen burden between primary and first recurrence tumors in 23 patients with HNSCC.
In this Oncotarget study, the authors characterized the mutational and neoantigen burden between primary and first recurrence tumors in 23 patients with HNSCC
The Van Tine Research Team concluded in their Oncotarget Research Output that there is a shifting neoantigen burden as there are unique neoantigens in primary tumors and different unique neoantigens in the recurrent/metastatic tumors.
The patients which have these neoantigens in shared genes are patients which have higher total numbers of neoantigens.
What is clear is that patients with neoantigens in these shared genes also tend to have increased duration of survival with disease.
The increase in neoantigens and duration of survival with disease tends to be associated with increased CD3 CD8 density in the tumor and CD8A expression.
This suggests that patients with these shared neoantigens are associated with increased CD8 T cell infiltration and increased cytotoxic activity, which extends the patient's life.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27907
Full text - https://www.oncotarget.com/article/27907/text/
Correspondence to - Brian A. Van Tine - [email protected]
Keywords - head and neck squamous cell carcinoma, neoantigens, mutational evolution, tumor relapse, immune cell infiltration
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



