Oncotarget Volume 11, Issue 27 published "Epigenetic feedback and stochastic partitioning during cell division can drive resistance to EMT" by Jia et al. which reported that Epithelial-mesenchymal transition (EMT) and its reverse process mesenchymal-epithelial transition are central to metastatic aggressiveness and therapy resistance in solid tumors.
While molecular determinants of both processes have been extensively characterized, the heterogeneity in the response of tumor cells to EMT and MET inducers has come into focus recently, and has been implicated in the failure of anti-cancer therapies.
Recent experimental studies have shown that some cells can undergo an irreversible EMT depending on the duration of exposure to EMT-inducing signals.
While the irreversibility of MET, or equivalently, resistance to EMT, has not been studied in as much detail, evidence supporting such behavior is slowly emerging.
Here, the authors' identify two possible mechanisms that can underlie resistance of cells to undergo EMT: epigenetic feedback in ZEB1/GRHL2 feedback loop and stochastic partitioning of biomolecules during cell division.
Identifying the ZEB1/GRHL2 axis as a key determinant of epithelial-mesenchymal plasticity across many cancer types, the authors' use mechanistic mathematical models to show how GRHL2 can be involved in both the above mentioned processes, thus driving an irreversible MET. This study highlights how an isogenic population may contain subpopulation with varying degrees of susceptibility or resistance to EMT, and proposes a next set of questions for detailed experimental studies characterizing the irreversibility of MET/resistance to EMT.
Dr. Herbert Levine from The Center for Theoretical Biological Physics at Rice University as well as The Department of Physics at Northeastern University and Dr. Mohit Kumar Jolly from The Centre for BioSystems Science and Engineering at The Indian Institute of Science said "Epithelial-Mesenchymal Transition (EMT) is a cell biological process involved in driving cancer metastasis and therapy resistance?the two grand clinically unsolved challenges."
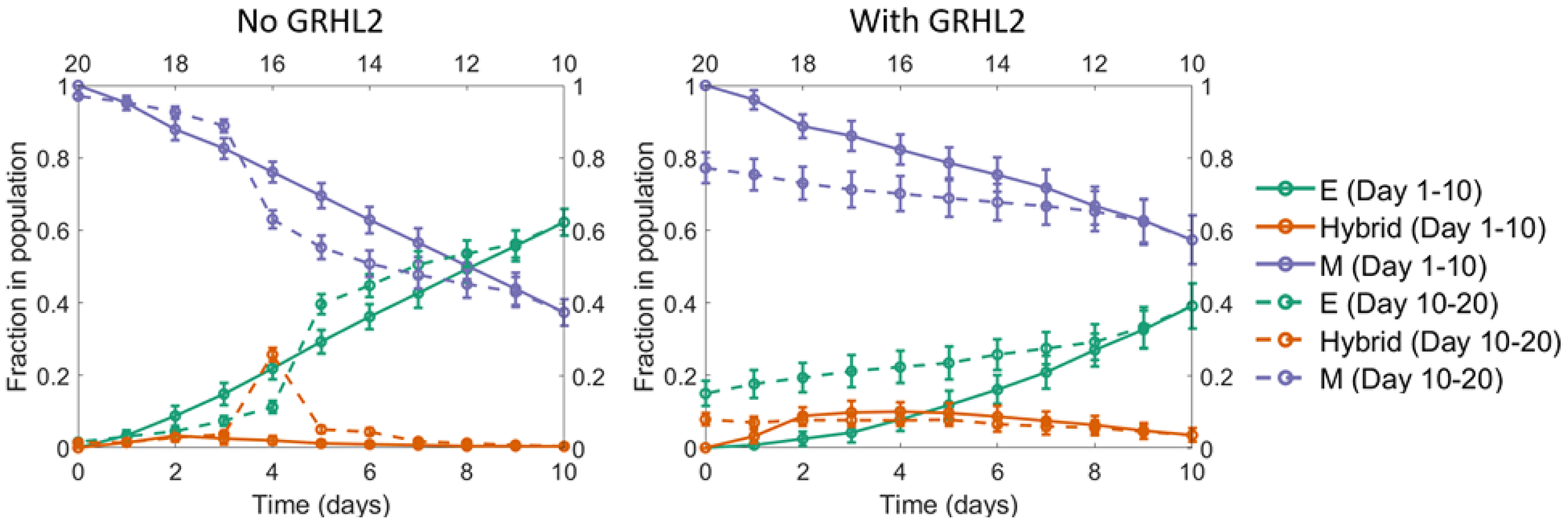
Figure 7: A seemingly irreversible MET at the population level. Starting with a population of mesenchymal cells on day 0 (all cells with very high value of the EMT inducer), the EMT inducer was withdrawn in fixed dosages each day for the first 10 days. As a result, a large fraction of cells in the population underwent MET. Day 11 onwards, fixed dosages of the EMT inducer were added each day for the next 10 days. In the absence of GRHL2 (left panel), the fraction of mesenchymal cells went back to nearly 100%, same as the value on day 0. However, in the presence of GRHL2 (right panel), ~15% of the cells in the population were epithelial on day 20. These cells thus represented a subpopulation that had undergone a MET that is irreversible at least on the time scale investigated here. The mean over 16 independent simulation runs is shown here. The error bars indicate the standard deviation over the independent runs.
These hybrid E/M phenotypes may drive collective cell migration as clusters of tumor cells and can be more aggressive than cells in pure epithelial or mesenchymal phenotypes.
Recent experiments decoding the dynamics of EMT/MET using live-cell imaging and/or induction and withdrawal of various EMT-inducing external signals such as TGF? or tuning the levels of EMT-specific transcription factors have provided important insights into the reversibility of EMT and MET. Cells induced to undergo EMT for shorter durations may revert to an epithelial state after withdrawal of the signal/stimulus.
However, similar investigations about the irreversibility of MET, or in other words, the resistance of epithelial cells to undergo EMT in response to EMT-inducing signals, remain to be done.
Here, the authors' propose two independent mechanism that may explain the resistance of epithelial tumor cells to undergo EMT:
- 1) epigenetic feedback mediated via GRHL2?an MET-inducing transcription factor ; and
- 2) stochastic partitioning of parent cell biomolecules among the daughter cells at the time of cell division.
Conversely, here, the authors' show that incorporating this epigenetic feedback loop acting on the inhibition of ZEB1 by GRHL2 can cause an irreversible MET. Cells undergoing irreversible MET may exhibit resistance in undergoing EMT when exposed to EMT-inducing signals.
The Levine/Jolly Research Team concluded in their Oncotarget Research Paper that their results offer mechanistic insights into two possible mechanisms that may drive varying degrees of susceptibility and resistance to undergoing EMT in response to an EMT-inducing signal in a given isogenic population.
"Results offer mechanistic insights into two possible mechanisms that may drive varying degrees of susceptibility and resistance to undergoing EMT in response to an EMT-inducing signal in a given isogenic population."
Future efforts should decode the molecular mechanisms of any such epigenetic feedback of GRHL2 on ZEB1 expression as well as track the distribution of molecules during cell divisions happening while cells are being induced to undergo EMT/MET. Moreover, this study calls for concerted efforts to map the single-cell dynamics of MET induction.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27651
Full text - https://www.oncotarget.com/article/27651/text/
Correspondence to - Herbert Levine - [email protected] and Mohit Kumar Jolly - [email protected].
Keywords - epithelial-mesenchymal transition, mesenchymal-epithelial transition, GRHL2, epigenetics, asymmetric cell division
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105





