Volume 11, Number 16 of @Oncotarget reported that through functional assays in vitro and in vivo, the authors show that GRK5 regulates cell cycle in a kinase-independent manner to promote RMS tumor cell growth.
Treatment of human RMS xenografts in mice with CCG-215022, a GRK5-selective inhibitor, results in reduced tumor growth and self-renewal in both major subtypes of RMS. GRK5 represents a novel therapeutic target for the treatment of RMS.
Dr. Eleanor Y. Chen from The Department of Pathology, University of Washington said, "Rhabdomyosarcoma (RMS) is the most common pediatric soft-tissue cancer."
"Rhabdomyosarcoma (RMS) is the most common pediatric soft-tissue cancer."
- Dr. Eleanor Y. Chen, The Department of Pathology, University of Washington
While previous studies have shown MEK, CDK4/6, and WEE1 as promising kinase targets for inhibiting tumor growth, druggable kinases against RMS self-renewal have been poorly characterized.
The study by Chen et al shows that chemical inhibition of glycogen synthase kinase 3 reduces ERMS tumor growth and self-renewal, demonstrating the therapeutic potential for targeting protein kinases that play a role in the regulation of RMS tumor growth and self-renewal.
While GRK5 has been extensively studied for its role in heart disease, the role of GRK5 in cancer pathogenesis is poorly characterized.
In non-small cell lung cancer and glioblastoma multiforme, GRK5 is highly expressed in primary patient specimens and depletion of GRK5 results in reduced cell growth.
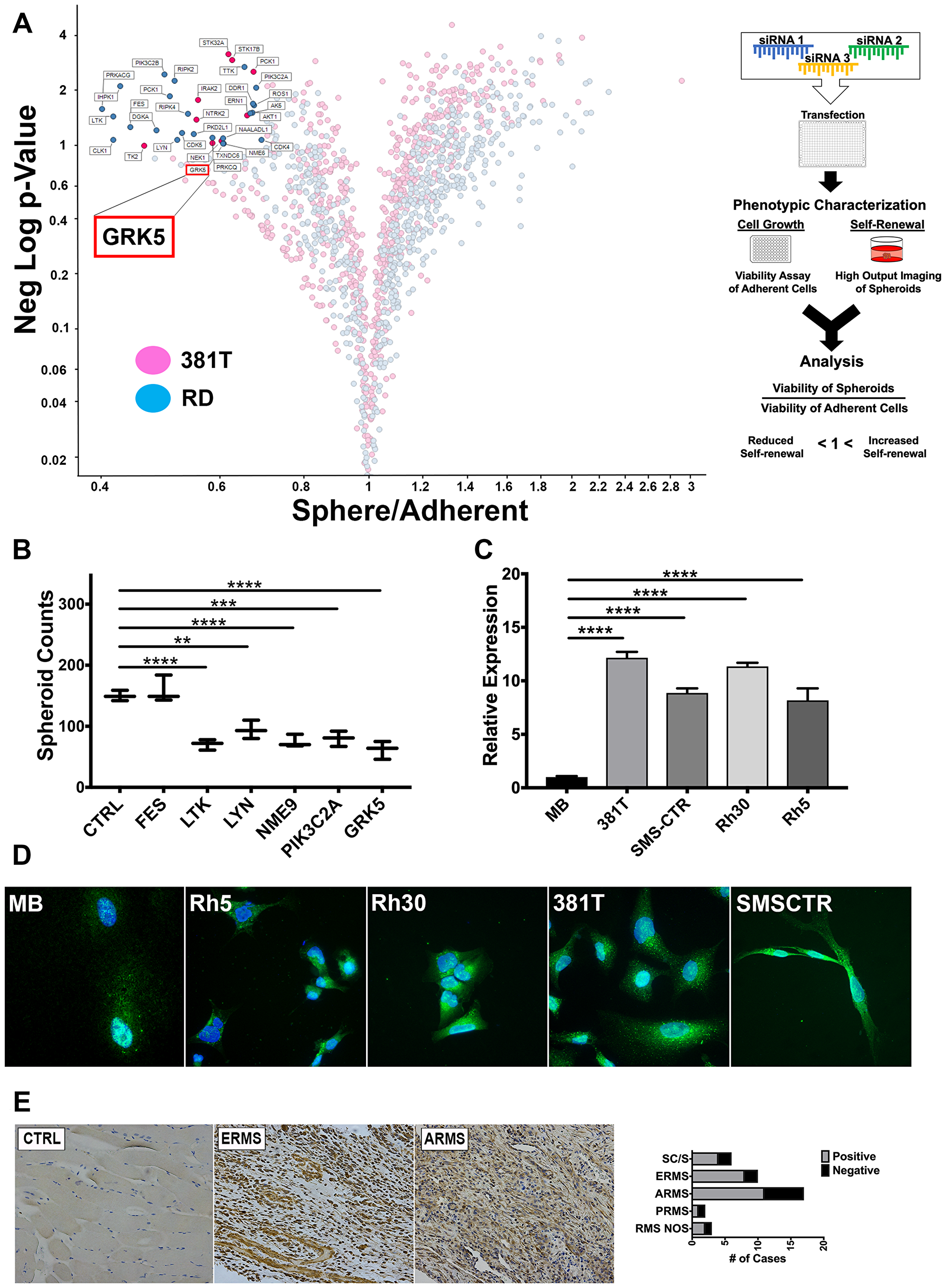
Figure 1: An siRNA library screen of the human kinome identifies GRK5 as a novel regulator of ERMS self-renewal. (A) Volcano plot illustrating candidate kinases identified from an siRNA library screen against the human kinome in ERMS cancer cell lines (381T and RD). Significant hits are indicated as having a p-value (Y-axis) of < 0.05 and a Sphere/Adherent viability ratio (X-axis) of < 1.0. Diagram on the right illustrates workflow and analysis used in the siRNA library screen. GRK5 is highlighted as being a candidate kinase identified from the screen. (B) Spheroid counts to assess self-renewal capacity was performed on CRISPR/Cas9 mediated knockout of top 6 candidate kinases (FES, LTK, LYN, NME9, PIK3C2A, GRK5). Error bars represent standard deviation of 3 technical replicates from an individual experiment that was repeated 3 times. (C) RT-PCR analysis of GRK5 expression in human myoblasts (MB) compared to a panel of RMS cancer cell lines (381T, SMS-CTR, RH30, RH5). Error bars represent standard deviation of 3 technical replicates from an individual experiment that was repeated 3 times. (D) Immunofluorescence images showing GRK5 staining in MB and RMS cancer cell lines (381T, SMS-CTR, Rh30, Rh5). (E) Immunohistochemistry of GRK5 in skeletal muscle control (CTRL) and representative primary ERMS and ARMS tumors. Summary of IHC for GRK5 in primary RMS tumors spotted on a tissue microarray is shown on the right. Spindle cell RMS (SC/S), embryonal RMS (ERMS), alveolar RMS (ARMS), pleomorphic RMS (PRMS), RMS not otherwise specified (RMS NOS). Two-tailed t-test; ** = p < 0.01; *** = p < 0.001, **** = p < 0.0001.
Loss of GRK5 in NSCLC and prostate cancer cell lines also results in cell cycle arrest.
Treatment of RMS xenografts with a selective GRK5 inhibitor, CCG-215022, results in a significant reduction of tumor growth, demonstrating the potential of GRK5 as a therapeutic target in RMS.
The Chen Research Team concluded in their Oncotarget Research Article, "With treatment options against RMS remaining relatively unchanged over the last 3 decades, there remains a need for more effective therapeutic targets. From a comprehensive siRNA library screen against the human kinome, we have identified GRK5 as a novel regulator of both RMS self-renewal and cell growth. Our functional characterization of GRK5 in vitro and in vivo demonstrates that GRK5 regulates ERMS cell growth in a kinase-independent manner and is essential for RMS self-renewal capacity. A GRK5 inhibitor, CCG-215022, recapitulates the loss-of-function effects of GRK5. Thus, our findings demonstrate the promise of GRK5 as a therapeutic target against RMS disease progression and relapse."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27562
Full text - https://www.oncotarget.com/article/27562/text/
Correspondence to - Eleanor Y. Chen - [email protected]
Keywords - rhabdomyosarcoma, self-renewal, GRK5, kinase, cell cycle
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


