Oncotarget published "Caloric restriction causes a distinct reorganization of the lipidome in quiescent and non-quiescent cells of budding yeast" which reported that at that point, the yeast culture starts to accumulate quiescent and non-quiescent cells.
Here, the authors purified the high- and low-density populations of quiescent and non-quiescent cells from the yeast cultures limited in calorie supply or not.
They found that caloric restriction, a geroprotective dietary intervention, alters the concentrations of many lipid classes through most of the chronological lifespan of the high- and low-density populations of quiescent and non-quiescent cells.
Based on these findings, the authors propose a hypothetical model for a caloric restriction-dependent reorganization of lipid metabolism in budding yeast's quiescent and non-quiescent cells.
They also discovered that caloric restriction creates lipidomic patterns of these cells that differ from those established by two other robust geroprotectors, namely the tor1Δ mutation and lithocholic acid.
Caloric restriction creates lipidomic patterns of these cells that differ from those established by two other robust geroprotectors
Dr. Vladimir I. Titorenko from The Concordia University said, "Culturing budding yeast Saccharomyces cerevisiae aerobically in a nutrient-rich liquid medium with 2% glucose as a single carbon source provides yeast enough calories to proliferate and survive."
The cell cycle arrest at the “START A” checkpoint coincides with the appearance of two cell populations in the yeast culture under non-CR conditions.
The major properties of the cellular quiescence program operating in yeast under non-CR conditions have been described as follows:
1) The program is initiated in response to nutrient deprivation and the onset of chronological aging,
2) The program integrates a series of cellular events; these events follow each other in a particular order and are under the tight control of a signaling network of yeast quiescence,
3) Specific genetic manipulations that alter the information flow down the program's consecutive steps can accelerate or decelerate the program, and
4) Akin to other programmed biological events, the cellular quiescence program is beneficial for the survival and stress resistance of a yeast cell's population.
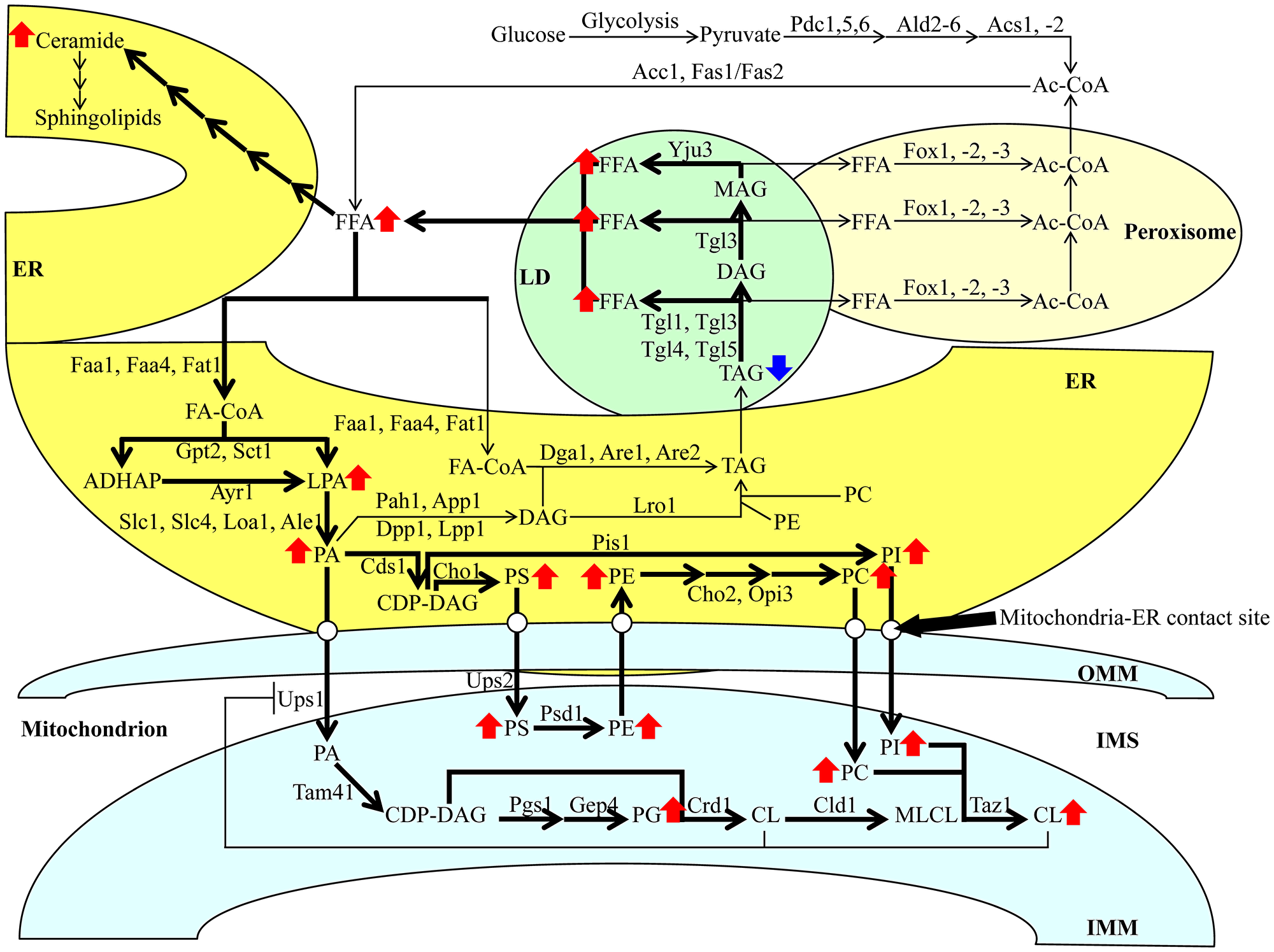
Figure 14: A model for a CR-dependent remodeling of lipid metabolism and transport in HD and LD cells. CR stimulates FFA formation because this low-calorie diet promotes the lipolysis of the neutral lipids TAG. CR also activates the incorporation of FFA into various phospholipid classes in the ER and mitochondria. Arrows next to the names of lipid classes indicate those whose concentrations are increased (red arrows) or decreased (blue arrows) in yeast cells cultured under CR conditions. The thickness of black arrows is proportional to the efficiency with which TAG is hydrolyzed, FFA is incorporated into phospholipids and phospholipids are transported from the ER to mitochondria via the mitochondria-ER contact sites. Abbreviations: All abbreviations are provided in the text and the legend for Figure 2.
Under non-CR conditions, the budding yeast cells that arrest their cell cycle and enter a G0 state become a Q cell population.
In contrast, the budding yeast cells under the same culturing conditions do not undergo cell cycle arrest; these cells form three different populations of NQ cells.
The population of Q cells in non-CR cultures includes unbudded cells of a uniform diameter; these cells have a thick cell wall, refract light if examined by phase-contrast microscopy and exhibit a high buoyant density.
The Titorenko Research Team concluded in their Oncotarget Research Output that "our recent studies provided evidence that CR creates a metabolic pattern of chronological aging delay that in budding yeast differs from the metabolic design established by the tor1Δ mutation and LCA [33]. These metabolites are soluble in aqueous solutions. Thus, it seems that different dietary, genetic and pharmacological geroprotectors differ in their ability to affect the water-insoluble lipidomes and water-soluble metabolomes of chronologically aging budding yeast."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28133
Correspondence to - Vladimir I. Titorenko - [email protected]
Keywords - cellular aging, cellular quiescence, caloric restriction, geroprotectors, lipids
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


