Oncotarget Volume 11, Issue 27 published "Australian experience of peptide receptor radionuclide therapy in lung neuroendocrine tumours" by Lim et al. which reported peptide receptor radionuclide therapy (PRRT) is an approved treatment modality for gastroenteropancreatic neuroendocrine tumours, although Phase III randomised clinical trial data is not available for NETs of other site of origin, in practice, PRRT is used more widely in clinical practice, based on its mechanism of targeting the somatostatin receptor.
A retrospective chart review of patients with TC and AC who received 177Lu-dotatate PRRT between January 2002 and June 2019 in six hospitals across Australia was undertaken.
- Forty-eight patients received a median of four 177Lu-dotatate treatments.
- The response rate to 177Lu-dotatate was 33%, with a median overall survival of 49 months, at a median follow up of 33 months.
- 177Lu-dotatate PRRT in patients with lung NETs is used in real world practice, where it appears well-tolerated with some efficacy.
Dr. Lisi Elizabeth Lim from The Department of Medical Oncology at Monash Health said "Neuroendocrine tumours (NETs) are uncommon malignancies, comprising 0.5% of all cancers."
Lung is the primary site for approximately 20?25% of NETs; conversely NETs comprises about 2% of all lung malignancies.
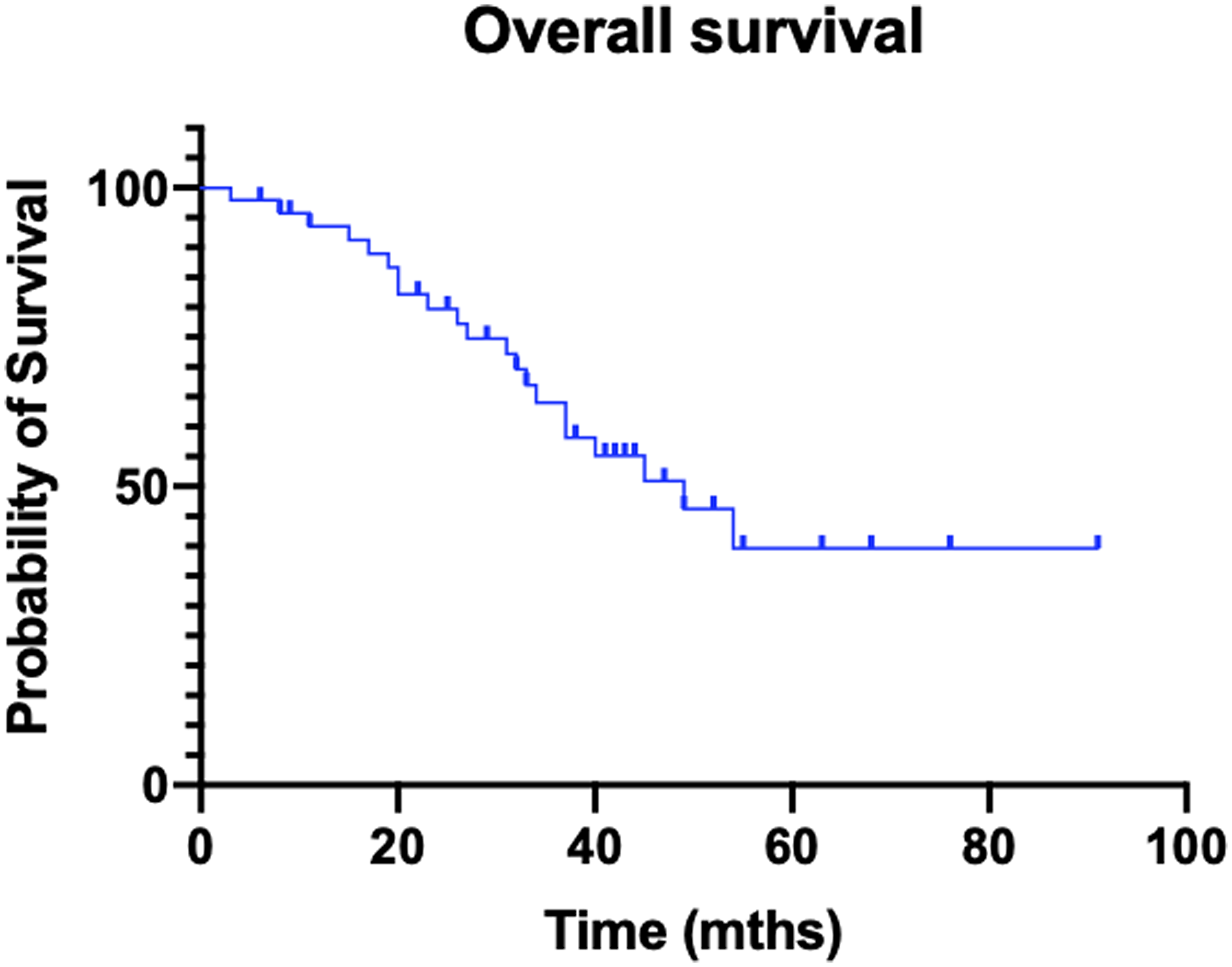
Figure 1: Kaplan?Meier curve of overall survival.
New trials have demonstrated that adequate numbers of patients can be recruited through global collaborations, both for protocols specific to lung NETs and those recruiting patients with NETs from a variety of sites.
PRRT is a firmly established treatment modality for advanced GEP NETs following the publication of the landmark NETTER-1 trial, where patients with progressive midgut NET were randomised to receive 177Lu-dotatate with ongoing octreotide long-acting repeatable therapy, or high dose octreotide LAR alone.
The significant benefit for PRRT in midgut NETs has provoked debate about whether randomised trials are required to prove its efficacy in NETs of other site of origin.
"The significant benefit for PRRT in midgut NETs has provoked debate about whether randomised trials are required to prove its efficacy in NETs of other site of origin."
NET consensus guidelines either omit specific comment on the use of PRRT in lung NETs, or state that imaging with SSTR-PET can assist in identifying patients who may benefit from PRRT. The Lim Research Team concluded in their Oncotarget Research Paper that further data will be forthcoming also from studies of PRRT in patients with SSTR-expressing tumours of histologies other than NET. The randomised phase II LUTHREE trial is inclusive of all SSTR positive tumour types, and is not restricted to NETs. The POLNETS trial is also extending the use of PRRT to paraganglioma and pheochromocytoma, in addition to advanced NETs of any site of origin.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27659
Full text - https://www.oncotarget.com/article/27659/text/
Correspondence to - Lisi Elizabeth Lim - [email protected].
Keywords - lung, carcinoid, atypical, neuroendocrine, peptide receptor radionuclide therapy
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105