Oncotarget published "Altered glucuronidation deregulates androgen dependent response profiles and signifies castration resistance in prostate cancer" which reported that Glucuronidation controls androgen levels in the prostate and the dysregulation of enzymes in this pathway is associated with castration resistant prostate cancer.
UDP-glucose dehydrogenase produces UDP-glucuronate, the essential precursor for glucuronidation, and its expression is elevated in prostate cancer. All pairs showed changes in UGDH and associated enzymes and metabolites that were consistent with those these authors found in an isogenic androgen dependent and CR LNCaP prostate cancer model.
Importantly, the knockdown of UGDH in both LNCaP AD and CR cells dramatically sensitized these cells to enzalutamide.
Importantly, the knockdown of UGDH in both LNCaP AD and CR cells dramatically sensitized these cells to enzalutamide
Dr. Melanie A. Simpson from The North Carolina State University as well as The University of North Carolina at Chapel Hill said, "Prostate cancer is the most frequently diagnosed cancer in US men, and the second most common cause of male cancer death."
Castration resistant recurrence following androgen deprivation therapy is a major cause of mortality in men with advanced prostate cancer. Tumors that recur after this treatment are highly aggressive and able to expand in conditions of low circulating androgens by one of several mechanisms, including aberrancies in androgen receptor expression or signaling, and upregulation of enzymes that control intratumoral androgen synthesis.
UGDH activity is also required to support biosynthetic processes: UDP-GlcA initiates specifically timed production of proteoglycans in the Golgi and is required to produce hyaluronan at the plasma membrane. Thus, although UGDH is a cytosolic enzyme, its product is needed for compartmentally segregated, energy-competitive processes, and the pools of UDP-GlcA must be tightly regulated.
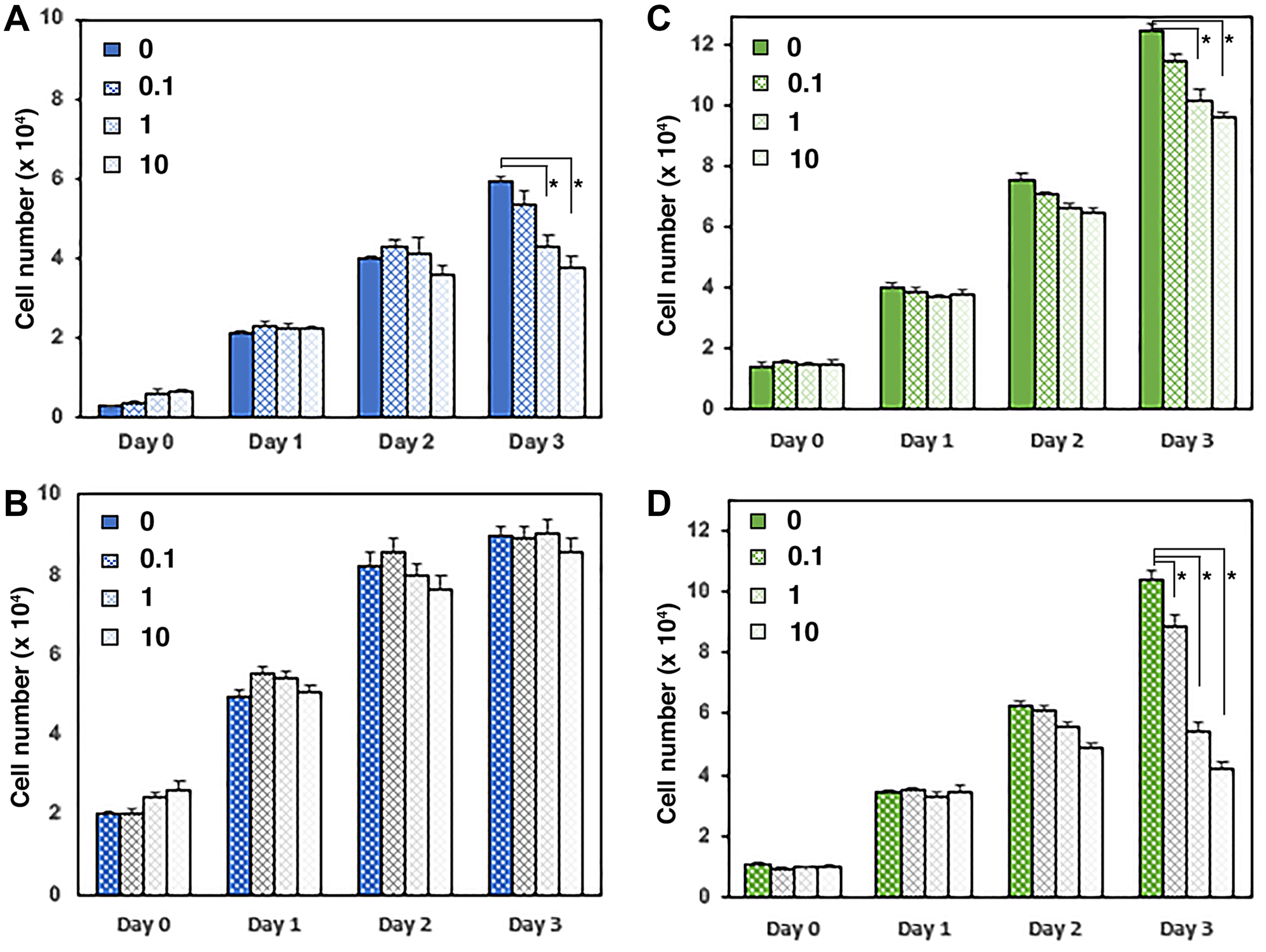
Figure 7: UGDH manipulation regulates concentration dependence of enzalutamide growth suppression. (A and B) LNCaP AD cells overexpressing UGDH (B) were compared to vector control (A) and to LNCaP CR cells with UGDH knocked down (C, Control; D, UGDH KD). Equal cell numbers were plated and treated for three days with the indicated concentrations of enzalutamide (in mM). Proliferating cells were quantified in a fluorescence plate reader using resazurin-resorufin conversion. Daily cell count was determined from a standard curve and represents the mean of four technical replicates ± SEM; *p < 0.05.
In contrast, UGDH is elevated in CR prostate cancer and the increase in those cells is associated with higher hyaluronan production and proteoglycan expression. Despite also having higher glucuronidation potential, glucuronide output is significantly reduced, likely indicating an intracellular prioritization scheme that is linked to precursor availability.
The Simpson Research Team concluded in their Oncotarget Research Output, "The ability to reverse castration resistance even partially by reducing UGDH expression and/or activity is significant. In particular, it would be valuable to determine whether UGDH inhibition combined with anti-androgen treatment at the start of therapy could prevent or delay onset of therapeutic resistance by improving initial response. Understanding the mechanisms underlying the alternate functions of UGDH and how it achieves metabolite partitioning will facilitate effective and more selective targeting."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28059
Full text - https://www.oncotarget.com/article/28059/text/
Correspondence to - Melanie A. Simpson - [email protected]
Keywords - prostate cancer, castration resistance, detoxification, UDP-glucose dehydrogenase, patient-derived xenografts
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105