Oncotarget published "A platform for locoregional T-cell immunotherapy to control HNSCC recurrence following tumor resection" which reported that the authors hypothesized that local delivery of tumor antigen-specific T-cells into the resection cavity following surgery would direct T-cells to residual antigens in the margins and draining lymphatics and present a platform for T-cell-targeted immunotherapy.
They loaded T-cells into a biomaterial that conformed to the resection cavity and demonstrated that it could release T-cells that retained their functional activity in-vitro, and in a HNSCC model in-vivo.
Locally delivered T-cells loaded in a biomaterial were equivalent in control of established tumors to intravenous adoptive T-cell transfer, and resulted in the systemic circulation of tumor antigen-specific T-cells as well as local accumulation in the tumor.
They demonstrate that adjuvant therapy with anti-PD1 following surgical resection was ineffective unless combined with local delivery of T-cells.
These Oncotarget data demonstrate that local delivery of tumor-specific T-cells is an efficient option to convert tumors that are unresponsive to checkpoint inhibitors to permit tumor cures.
These Oncotarget data demonstrate that local delivery of tumor-specific T-cells is an efficient option to convert tumors that are unresponsive to checkpoint inhibitors to permit tumor cures.
Dr. Michael J. Gough from The Providence Portland Medical Center said, "Tumor-positive surgical margins are one of the most important risk factors for recurrence following surgical resection of HNSCC."
Therefore, it would potentially be advantageous to rapidly direct transferred T-cells to the tumor-draining lymph node for in-vivo expansion, before cross-presentation of tumor-derived material is lost. One approach to achieve this is to load the T-cells into the tumor-draining lymphatics via local delivery, to give the maximum chance of meeting cognate antigen on antigen-presenting cells to permit in-vivo expansion.
The tumor resection cavity can be a poorly defined and disrupted site for the local delivery of cells and drugs.
Importantly, T-cells can be incorporated into biomaterials for locoregional delivery, and combined with additional T-cell activating agents to improve tumor control.
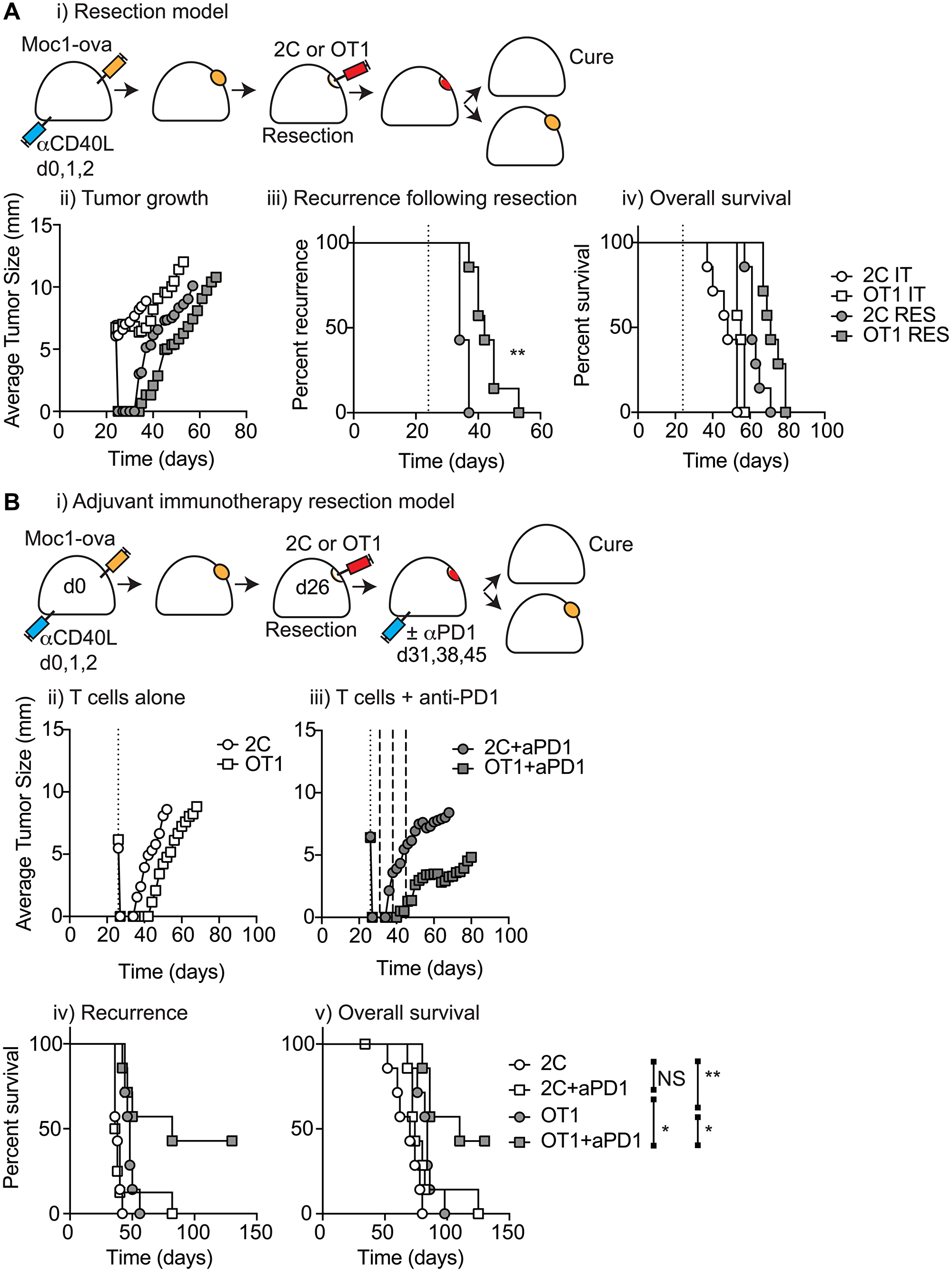
Figure 5: T-cell biomaterial control of recurrence following surgical resection. T(A) (i) MOC1-ova tumors were established in immune competent C57BL/6 mice and underwent IT administration of tumor-specific OT1 T-cells or non-specific 2C T-cells in 30 μl of Matrigel into the tumor, or subcomplete resection and administration of the same biomaterials into the resection cavity. Graphs show (ii) average tumor size for all groups (iii) recurrence for resection groups, and (iv) overall survival of all treated mice. (B) (i) Immune competent mice bearing MOC1-ova tumors underwent surgical resection and administration of T cell biomaterials into the resection cavity as per a). Mice were randomized to receive no further treatment or 3 weekly doses of adjuvant anti-PD1 starting 5d following resection. Graphs show average tumor size for (ii) T-cells alone or (iii) T-cells plus adjuvant anti-PD1. Graphs show (iv) recurrence following resection, and (v) overall survival of treated mice. Abbreviation: NS: not significant. *p < 0.05, **p < 0.01.
They propose that biomaterial delivery of T-cells to the resection site will naturally deliver these cells into contact with residual cancer cells or into the lymphatic drainage for expansion and recirculation to prevent local recurrence.
Importantly, they show that adjuvant therapy with anti-PD1 fails to impact local recurrence unless combined with local delivery of tumor-specific T-cells, demonstrating that this approach has the potential to help patients with poor pre-existing anti-tumor reactivity.
The Gough Research Team concluded in their Oncotarget Research Output, "these data demonstrate a potential therapy for patients with poorly infiltrated tumors with a high risk of recurrence following surgical resection. Further work is needed to develop a biomaterial for clinical translation. A range of potential biomaterial options are available for direct depot injection of immunotherapies alone, or in surgical settings (reviewed in [11, 37, 38]). In addition, it will be valuable to evaluate additional components to support T-cells and control the local inflammatory environment to maximize T-cell control of residual disease."
DOI - https://doi.org/10.18632/oncotarget.27982
Full text - https://www.oncotarget.com/article/27982/text/
Correspondence to - Michael J. Gough - [email protected]
Keywords - head and neck cancer, T-cell, immunotherapy, biomaterial, intratumoral
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



