The cover for issue 77 of Oncotarget features Figure 2, "In mouse PCTs, deletion of hs3b-4 does not affect the pattern of chromosomal translocations but significantly increases targeting of Sμ for T(12;15)," by Kovalchuk, et al.
To elucidate the role of the Igh3RR in tumorigenesis, we induced PCTs in Bcl-xL-transgenic mice deficient for the major Igh3RR enhancer elements, hs3b and hs4. Cre-mediated deletion of the Igh3RR resulted in gradual reduction of Myc expression, loss of proliferative activity and increased cell death, confirming the necessity of the Igh3RR for Myc deregulation by T.
Dr. Alexander L. Kovalchuk from the Virology and Cellular Immunology Section, Laboratory of Immunogenetics, National Institute of Allergy and Infectious Diseases, at the National Institutes of Health, in Rockville, MD, USA said, "the two major regulatory elements in the Igh locus are the E intronic enhancer that promotes V J recombination in developing B-cells, and an enhancer cluster located downstream of all constant gene segments, termed the 3 regulatory region, and recently classified as a superenhancer, which has a crucial functional role."
There are numerous mouse models that result in the development of B-cell or plasma cell tumors with the T translocation. By combining several mutations, it was also possible to obtain lymphomas with peripheral B-cell phenotype in which Myc was joined with an Igh switch region sequence and the E enhancer was deleted, leaving Myc under the control of the Igh3RR alone.
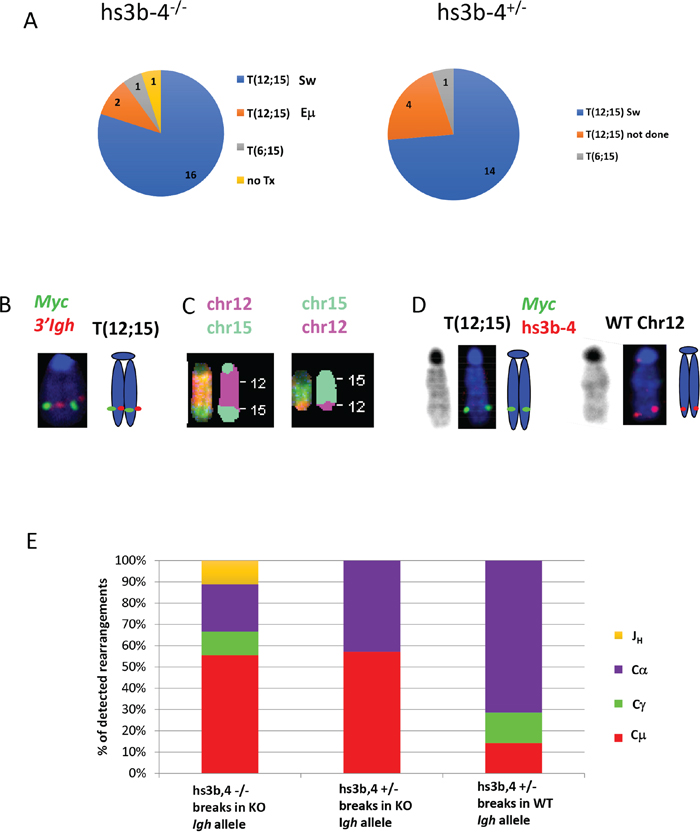
Figure 2: In mouse PCTs, deletion of hs3b-4 does not affect the pattern of chromosomal translocations but significantly increases targeting of Sμ for T(12;15). (A) Frequency of PCT tumors on hs3b-4-/- (left) or hs3b-4+/- (right) background with T(12;15) breakpoints within Igh switch regions; with T(12;15) breakpoints upstream of Eμ; with variant translocations T(6;15) or without Ig/Myc translocations. Chart shows predominance of tumors with T(12;15) with breakpoints in switch regions in both groups. (B) Cytogenetic findings in regards to T(12;15) translocations in the hs3b-4-/- group. Left – FISH with Igh probe (red) and Myc probe (green). Signals co-localize at the telomeric portion of Chr. 12. Right - graphic representation. (C) SKY images of Chr 12;15 and 15;12. (D) Targeting hs3b-4 KO allele by T(12;15) in hs3b-4+/- tumors. FISH using simultaneous staining with Myc (green) and hs3b-4 probes (red). The hs3b-4 probe hybridizes only to the intact WT Chr 12 (right). The Myc probe hybridizes to normal Chr 15, Chr T15;12 (both not shown here but can be found on Supplementary Figure 2) and to longer translocated Chr T(12;15) that has deletion in the Igh3'RR (left). Right - graphic representation. Left – DAPI staining. (E) Distribution of Igh/Myc breakpoints within JH, Cμ, Cγ (combined Cγ2a and b) and Cα. Shows prevalence of Cμ breakpoints in the hs3b-4-/- group and Cα in the WT group.
The function of Igh3RR individual enhancer units changes through different stages of B-cell differentiation with hs1,2 enhancers sequentially gaining higher activity during the GC and plasma cell stages.
The Alexander L. Kovalchuk research team concluded that hs3b-4 enhancer is crucial for synapsis with various parts of the Igh locus at the initiation of translocations in mature B-cells and for subsequent Myc deregulation, but then becomes dispensable once a translocation-positive precursor cell differentiates to the plasma cell stage and went on to say "upon plasma cell transition, hs3a and hs1,2 enhancers acquire the leading role in regulating the activity of the Igh locus."
The question remains - If such a hypomorphic Igh3RR with a partial deletion can support Myc deregulation in plasma cell tumors, is it then possible that the complete hs1,2,3a,3b,4 combination of Igh3RR enhancers is no longer essential in established PCTs?
Full text - https://doi.org/10.18632/oncotarget.26160
Correspondence to - Alexander L. Kovalchuk - [email protected]
Keywords - immunoglobulin, Myc, translocation, PCT