Corrections:
Correction: First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL
Metrics: PDF 3414 views | ?
1 Division of Hematology, Ohio State University Wexner Medical Center, Columbus, OH, USA
2 Division of Medical Oncology, University of Washington, Seattle, WA, USA
3 Division of Oncology, Washington University, St. Louis, MO, USA
4 Willamette Valley Cancer Institute and Research Center, US Oncology, Eugene, OR, USA
5 Center for Hematologic Malignancies, Oregon Health & Science University, Portland, OR, USA
6 Stanford Cancer Institute, Stanford School of Medicine, Stanford, CA, USA
7 Sir Charles Gairdner Hospital, Perth, WA, Australia
8 Department of Haematology, Princess Alexandra Hospital, Brisbane, QLD, Australia
9 Early Clinical Development, Genentech, Inc., South San Francisco, CA, USA
10 Blood Cancer Research Program, Sarah Cannon Research Institute, Nashville, TN, USA
Published: June 04, 2019
This article has been corrected: Due to errors in figure preparation, the data for Table 3 was listed incorrectly. It has now been updated with corrected numbers (see text in Table 3) and the resulting graphs (Figure 2A and 2B) have been updated as well. In addition, the reference list for this paper was accidentally alphabetized during the production process, while citation numbers within the article remained according to first-mention. In this correction, the entire references list has been corrected. We include the reference list in their order of mention so that the citation number in the article corresponds to the reference number in the list.
Original article: Oncotarget. 2018; 9:13023–13035. https://doi.org/10.18632/oncotarget.24310
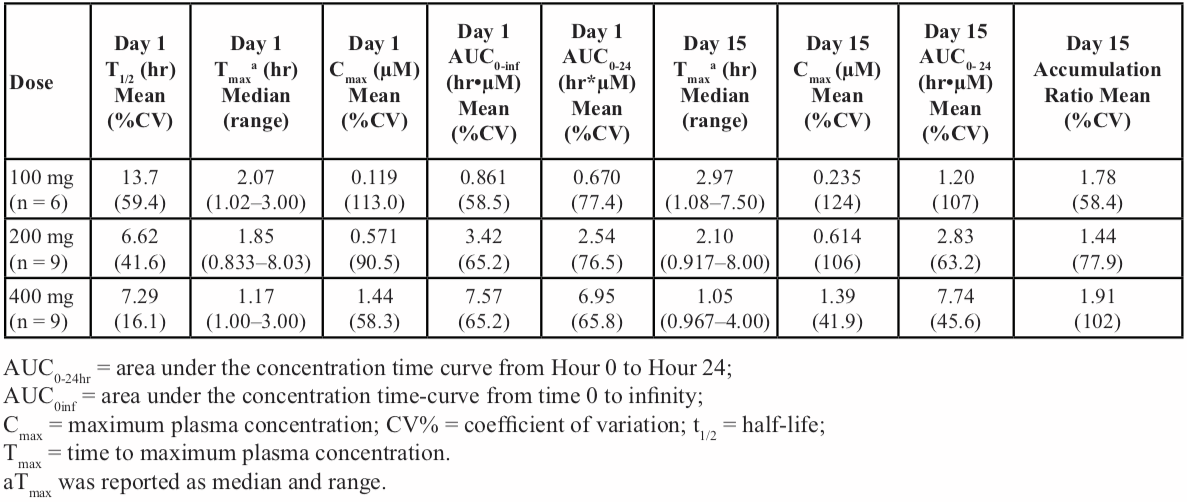
Table 3: Summary of pharmacokinetics parameters for GDC-0853 on day 1 and day 15 (cohorts 1, 2, and 3, with 100, 200, and 400-mg GDC-0853, respectively
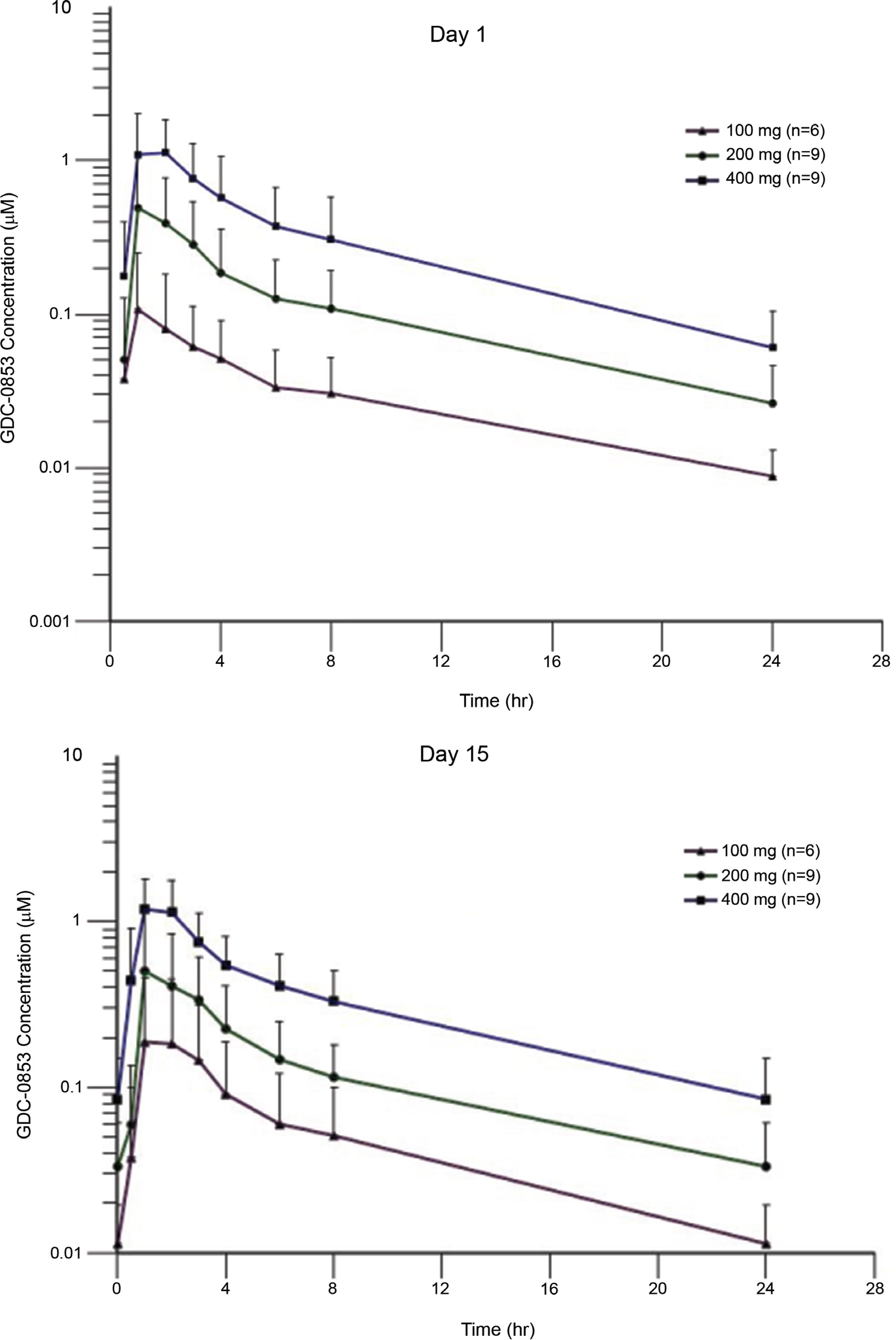
Figure 2: Pharmacokinetics profile of GDC-0853. Mean (±SD) GDC-0853 concentration-time profile on day 1 (A) and day 15 (B) after 100, 200, or 400 mg dose of GDC0853.
- 1. Maloney DG, Grillo-Lopez AJ, White CA, et al. IDECC2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997; 90:2188–2195.
- 2. Jaglowski SM, Byrd JC. Rituximab in chronic lymphocytic leukemia. Semin Hematol. 2010; 47:156–169.
- 3. Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibi- tor of phosphatidylinositol 3 kinase p110delta, for relapsed/ refractory chronic lymphocytic leukemia. Blood. 2014; 123:3390–7.
- 4. Kahl BS, Spurgeon SE, Furman RR, et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/ refractory mantle cell lymphoma (MCL). Blood. 2014; 123:3398–3405.
- 5. Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activ- ity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013; 31:88–94.
- 6. Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013; 369:1278- 1279.
- 7. Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014; 123:3406–3413.
- 8. Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014; 370:1008–1018.
- 9. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibru- tinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013; 369:507–516.
- 10. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015; 372:1430–1440.
- 11. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013; 369:32–42.
- 12. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatu- mumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014; 371:213–223.
- 13. Jiang A, Craxton A, Kurosaki T, Clark EA. Different pro- tein tyrosine kinases are required for B cell antigen receptor- mediated activation of extracellular signal regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J Exp Med. 1998; 188:1297–1306.
- 14. Petro JB, Khan WN. Phospholipase C-gamma 2 couples Bruton’s tyrosine kinase to the NF-kappaB signaling pathway in B lymphocytes. J Biol Chem. 2001; 276:1715–1719.
- 15. Bajpai UD, Zhang K, Teutsch M, Sen R, Wortis HH. Bruton’s tyrosine kinase links the B cell receptor to nuclear factor kap- paB activation. J Exp Med. 2000; 191:1735–1744.
- 30. Erickson RI, Schutt LK, Tarrant J, et al. BTK small mole- cule inhibitors induce a distinct pancreatic toxicity in rats. J Pharmacol Exp Ther. 2017; 360:226–238.
- 31. Reiff SD GD, Mantel R, Smith L, Cheney C, Johnson AJ, Byrd JC, Woyach JA. Evaluation of the novel Bruton′s tyro- sine kinase (BTK) inhibitor GDC-0853 in chronic lympho- cytic leukemia (CLL) with wild type or C481S mutated BTK. J Clin Oncol. 2016; 34:abstr 7530.
- 32. Johnson AR, Kohli PB, Katewa A, et al. Battling Btk Mutants With Noncovalent Inhibitors That Overcome Cys481 and Thr474 Mutations. ACS Chem Biol. 2016; 11:2897–907.
- 33. Todd J, Freese B, Lu A, et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem. 2007; 53:1990–1995.
- 34. Fischer SK, Joyce A, Spengler M, et al. Emerging technolo- gies to increase ligand binding assay sensitivity. AAPS J. 2015; 17:93–101.
- 35. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leuke- mia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute- Working Group 1996 guidelines. Blood. 2008; 111:5446–5456.
- 36. Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lympho- cytic leukemia. J Clin Oncol. 2012; 30:2820–2822.
- 16. Spaargaren M, Beuling EA, Rurup ML, et al. The B cell antigen receptor controls integrin activity through Btk and PLCgamma2. J Exp Med. 2003; 198:1539–1550.
- 17. Doyle SL, Jefferies CA, Feighery C, O’Neill LA. Signaling by Toll-like receptors 8 and 9 requires Bruton’s tyrosine kinase. J Biol Chem. 2007; 282:36953–36960.
- 18. Hasan M, Lopez-Herrera G, Blomberg KE, et al. Defective Toll-like receptor 9-mediated cytokine production in B cells from Bruton’s tyrosine kinase-deficient mice. Immunology. 2008; 123:239–249.
- 19. Kil LP, de Bruijn MJ, van Nimwegen M, et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood. 2012; 119:3744–3756.
- 20. Kil LP, de Bruijn MJ, van Hulst JA, Langerak AW, Yuvaraj S, Hendriks RW. Bruton’s tyrosine kinase mediated signal- ing enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia. Am J Blood Res. 2013; 3:71–83.
- 21. Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014; 123:1207–1213.
- 22. Woyach JA, Furman RR, Liu TM, et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N Engl J Med. 2014; 370:2286–2294.
- 23. Burger JA, Landau DA, Taylor-Weiner A, et al. Clonal evolu- tion in patients with chronic lymphocytic leukaemia devel- oping resistance to BTK inhibition. Nat Commun. 2016; 7:11589.
- 24. Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015; 1:80–87.
- 25. Chiron D, Di Liberto M, Martin P, et al. Cell-cycle repro- gramming for PI3K inhibition overrides a relapsespecific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014; 4:1022–1035.
- 26. Woyach JA, Ruppert AS, Guinn D, Lehman A, Blachly JS, Lozanski A, Heerema NA, Zhao W, Coleman J, Jones D, Abruzzo L, Gordon A, Mantel R, et al. BTKC481S- Mediated Resistance to Ibrutinib in Chronic Lymphocytic Leukemia. J Clin Oncol. 2017; 35:1437–43.
- 27. Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015; 125:2062–2067.
- 28. Martin P, Maddocks K, Leonard JP, et al. Postibrutinib out- comes in patients with mantle cell lymphoma. Blood. 2016; 127:1559–1563.
- 29. Young W, Crawford J. Discovery of GDC-0853: A highly potent, selective, and non-covalent BTK inhibitor. Paper pre- sented at: Annual meeting of the American Chemical Society. 2016; (pp. 13–17). San Diego, CA.
- 37. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–586.
- 38. Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013; 12:229–243.
- 39. O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukae- mia or small lymphocytic lymphoma: an open-label, multi- centre, phase 1b/2 trial. Lancet. Oncol. 2014; 15:48- 58.
- 40. Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP- 196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016; 374:323–332.
- 41. Thompson PA, O’Brien SM, Wierda WG, et al. Complex karyotype is a stronger predictor than del(17p) for an infe- rior outcome in relapsed or refractory chronic lymphocytic leukemia patients treated with ibrutinib-based regimens. Cancer. 2015; 121:3612–3621.
- 42. Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leu- kemia cell survival and tissue homing in vitro and in vivo. Blood. 2012; 119:1182–1189.
 All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
PII: 27011
