Oncotarget Volume 11 Issue 8 reported that while it is known that cancer cells require one-carbon and FAD-dependent mitochondrial metabolism to sustain cell proliferation, the role of SLC25A32 in cancer cell growth remains unexplored.
Si RNA-mediated knock-down and CRISPR-mediated knock-out of SLC25A32 in cancer cells of different origins, resulted in the identification of cell lines sensitive and resistant to SLC25A32 inhibition.
Treatment of cells with the FAD precursor riboflavin and with GSH rescues cancer cell proliferation upon SLC25A32 down-regulation.
Dr. Sven Christian from Bayer AG, Drug Discovery, in Berlin Germany said "Altered tumor metabolism is described as a hallmark of tumor biology and is essential for the adaptation of tumor cells to their specific needs, e. g. a higher demand for energy and macromolecules."
"Altered tumor metabolism is described as a hallmark of tumor biology and is essential for the adaptation of tumor cells to their specific needs, e. g. a higher demand for energy and macromolecules."
- Dr. Sven Christian, Bayer AG, Drug Discovery
Due to the glycolytic switch of tumor cells, mitochondrial biology and especially mitochondrial oxidative phosphorylation have been considered of minor importance in cancer biology.
Although the outer mitochondrial membrane was shown to be relatively permeable, the inner mitochondrial membrane is comparatively impermeable and consequently contains several transporter proteins to overcome such a physical barrier.
The SLC25 family consists of 53 members localized at the inner mitochondrial membrane that transport a wide range of molecules involved in essential mitochondrial processes such as redox balance, the urea and citric acid cycles, oxidative phosphorylation, DNA maintenance and iron metabolism.
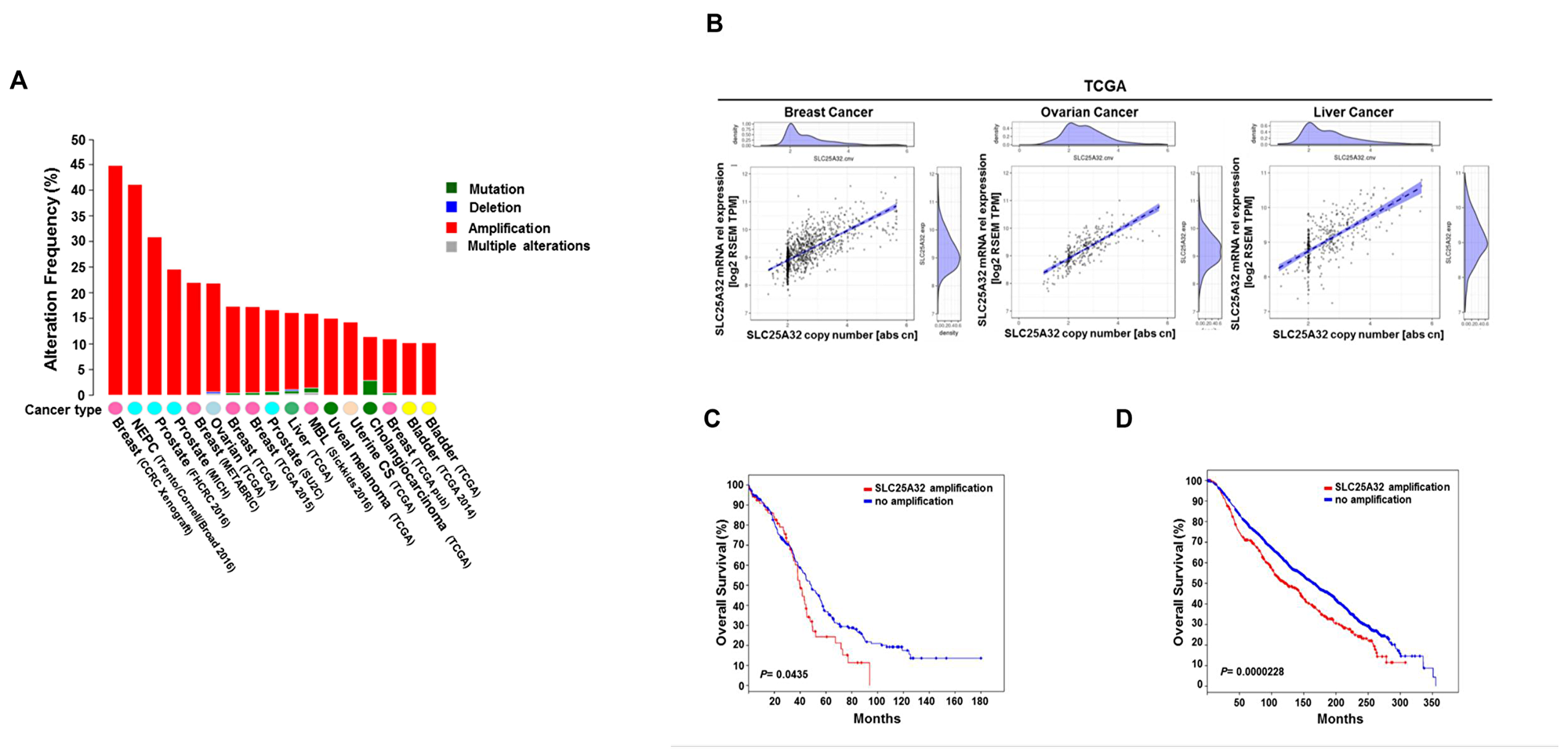
Figure 1: Genetic alterations of SLC25A32 reduce survival of cancer patients. (A) Representation of SLC25A32 genetic alterations across different cancers (www.cbioportal.org). (B) Spearman's rank correlation between SLC25A32 mRNA expression (RSEM TPM) and somatic copy number in breast cancer (1075 sample; P < 0.05), ovarian cancer (300 sample; P = 0.0.05) and liver cancer (364 sample; P = 0.05) in patient samples of TCGA. Each dot represents a tumor sample of one particular patient. The dotted line represents a linear regression line and the blue area around the fitted line shows the 95% confidence intervals. (C) Median overall survival data of ovarian carcinoma patients with SLC25A32 amplification (67 cases) and no amplification (241 cases). Median survival difference between the two groups is statistically significant (P = 0.0435). (D) Median overall survival data obtained from breast carcinoma patients with SLC25A32 amplification (407 cases) and no amplification (1459 cases) are presented. Median survival difference between the two groups is statistically significant (P = 0.0000228)
Uncoupling proteins are transporting protons across the mitochondrial membrane and thus, uncouple the transport from ATP generation.
In support of this, yeast lacking the mitochondrial FAD transporter FLX1, could be rescued by human SLC25A32 expression, suggesting that this transporter may also transport FAD across the inner membrane.
The Christian Research Team concluded in their Oncotarget Research Article that the data suggests that inhibition of SLC25A32 is anti-proliferative in a subset of tumor cell lines, at least partially by an increase of reactive oxygen species as a result of a malfunctional FAD-dependent enzymes such as SDH and that resistant cell line can compensate for the loss by the availability of higher reducing capacities. The study validates the role of SLC25A32 as a novel cancer target involved in the regulation of FAD-dependent mitochondrial metabolism. Molecular targeting of SLC25A32 using a single agent or in combination with ROS-inducing therapies could be an effective clinical strategy to successfully treat cancer patients.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27486
Full text - https://www.oncotarget.com/article/27486/text/
Correspondence to - Sven Christian - [email protected]
Keywords - transporter, mitochondria, metabolism, ROS, FAD
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.impactjournals.com/ or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


