Oncotarget published "Occurence of RAS reversion in metastatic colorectal cancer patients treated with bevacizumab" which reported that a disappearance of RAS mutations in the plasma of about 50% of mCRCs treated with bevacizumab-based chemotherapy has been reported.
Using next-generation sequencing and real-time PCR approaches, these authors characterized the primary tumor and paired liver metastases in 28 RAS mutant mCRCs.
RAS mutant alleles are at the same percentage in PT and liver metastases in the control group, while a significant reduction of the level of RAS mutations was detected in 57.1% of cases in group 1 and in 8.3% of cases in group 2. Differences among groups are statistically significant.
Most mCRC patients treated with bevacizumab-containing regimens experience a strong reduction of RAS mutant cells, suggesting bevacizumab as particularly active against RAS mutant cells.
This Oncotarget finding might have potential therapeutic implications, as anti-EGFR could be reconsidered in primarily RAS mutant patients reverted to a wild-type status after bevacizumab exposure.
This Oncotarget finding might have potential therapeutic implications, as anti-EGFR could be reconsidered in primarily RAS mutant patients reverted to a wild-type status after bevacizumab exposure.
Dr. Samantha Epistolio from The EOC said, "The overall survival (OS) for patients with metastatic colorectal cancer (mCRC) has markedly improved within the last 2 decades, reaching approximately 30 months."
Thus, it is crucial to perform an extended RAS and BRAF mutation analysis before considering EGFR inhibitors in mCRC patients.
In first-line, data support the use of anti-EGFR MoAbs in patients with left-sided, RAS and BRAF wild-type tumors, whilst bevacizumab is preferred in combination with chemo-doublets or -triplet in RAS/BRAF mutated tumors or RAS/BRAF wild-type right-sided primaries.
More recently, two intriguing studies showed that cetuximab sensitivity might be restored either in RAS/BRAF wild-type mCRC patients who acquired resistance to cetuximab-based therapy in first-line therapy or in those with primarily RAS mutated tumors treated with bevacizumab-containing regimens in second-line.
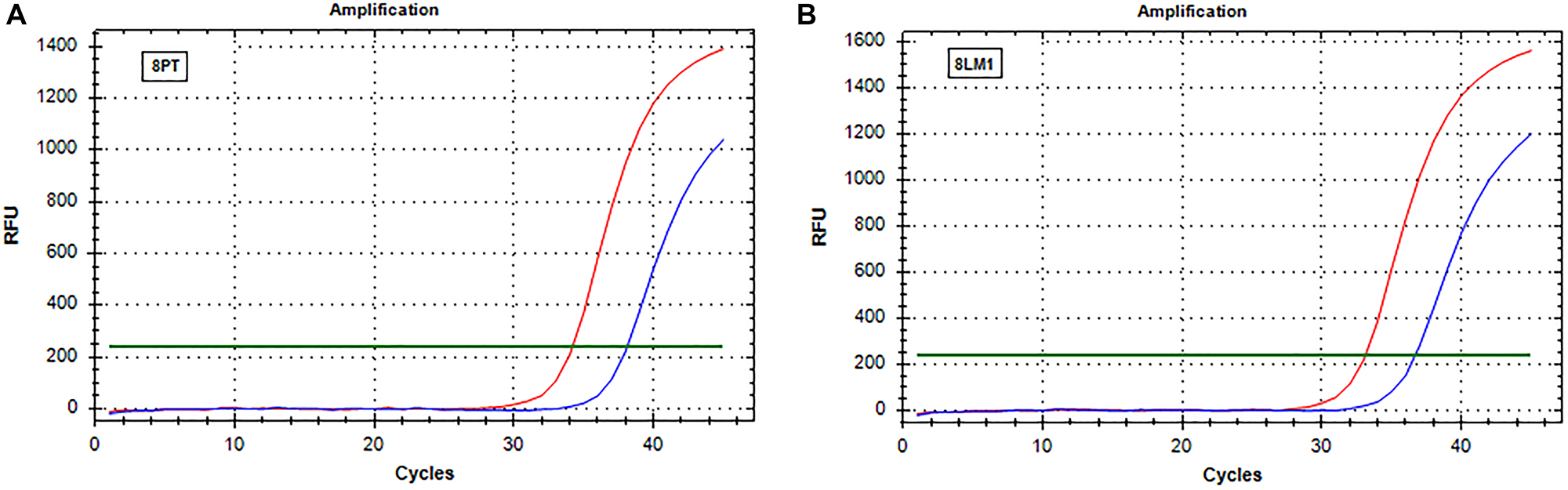
Figure 2: Real-time PCR (SensiScreen™) amplification curves of patient 8 (group 2). X-axis reports real-time PCR cycles and Y-axis reports relative fluorescence unit (RFU). In red is represented the amplification of the reference gene and in blue the amplification of the specific mutation (G13D). (A) Curves obtained from amplification of DNA extracted from sample 8PT; (B) Curves obtained from amplification of DNA extracted from sample 8LM1. Abbreviations: 8LM1, liver metastasis sample (patient 8); 8PT, primary tumor sample (patient 8); RFU, relative fluorescence unit.
These studies may lead to the hypothesis that anti-angiogenic agents through action on RAS mutated cells could revert tumors from RAS mutant to RAS wild-type status, which theoretically could lead to the possibility to treat these patients with anti-EGFR MoAbs, otherwise precluded.
To substantiate these findings at tissue level, here they analyzed molecular changes in RAS mutated mCRC patients treated with chemotherapy alone or in combination with bevacizumab, by examining tumor tissue samples before and after the systemic therapy using two independent methodologies, next-generation sequencing and real-time PCR.
The Epistolio Research Team concluded in their Oncotarget Research Output, "this retrospective observational study, strongly suggests the existence of a link between bevacizumab exposure and RAS status changes in mCRC patients. In addition to other reports using liquid biopsies, our findings on tissue samples corroborate the hypothesis that bevacizumab could revert RAS mutant mCRC to a wild-type pattern, conceptually opening to the possibility to treat with anti-EGFR MoAbs mCRC patients otherwise excluded based on initial RAS mutated status."
DOI - https://doi.org/10.18632/oncotarget.27965
Full text - https://www.oncotarget.com/article/27965/text/
Correspondence to - Samantha Epistolio - [email protected]
Keywords - RAS mutations, bevacizumab, metastatic colorectal cancer, next-generation sequencing
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105





