Oncotarget published "Loss of DRO1/CCDC80 in the tumor microenvironment promotes carcinogenesis" which reported that tumors are composed of the tumor cells and the surrounding microenvironment. Both are closely interwoven and interact by a complex and multifaceted cross-talk which plays an integral part in tumor initiation, growth, and progression.
The aim of the present study was to investigate whether Dro1/Ccdc80's tumor suppressive function is tumor-cell-autonomous. Expression of Dro1/Ccdc80 in cancer cells had no effect on both colon tumor development in ApcMin/+ mice and formation of xenograft tumors.
Moreover, stromal Dro1/Ccdc80 inactivation facilitated formation of intestinal epithelial organoids. Expression analyses showed Dro1/Ccdc80 to be significantly down-regulated in murine gastric cancer associated fibroblasts, in ApcMin/+ colon tumor primary stromal cells and in microdissected stroma from human colorectal cancer compared to normal, non-tumor stroma.
Dr. Jessica I. Christian from The Ludwig Maximilian University of Munich said, "Dro1/Ccdc80 has been identified as a tumor suppressor of colorectal, thyroid, and ovarian cancer."
In ApcMin/+ mice ubiquitous inactivation of Dro1/Ccdc80 results in early death, a significant increase in the colonic tumor load, and the regular formation of adenocarcinoma in the colon.
In ApcMin/+ mice ubiquitous inactivation of Dro1/Ccdc80 results in early death, a significant increase in the colonic tumor load, and the regular formation of adenocarcinoma in the colon.
The stromal compartment is known to stimulate tumor cell proliferation and mediate evasion of tumor cells from apoptosis, promote continuous angiogenesis, and contribute to the invasive and metastatic process. Moreover, tumor stromal cells convey drug sensitivity as well as therapeutic resistance and provide prognostic and response-predictive information. To date, the tumor suppressor role of Dro1/Ccdc80 in vivo has always been addressed by ubiquitous gene inactivation in mice.
For the study of tumorigenesis this approach implicates Dro1/Ccdc80 deficiency in both the tumor parenchyma and the tumor microenvironment. The aim of the present study was to investigate whether Dro1/Ccdc80's tumor suppressive function is tumor-cell-autonomous.
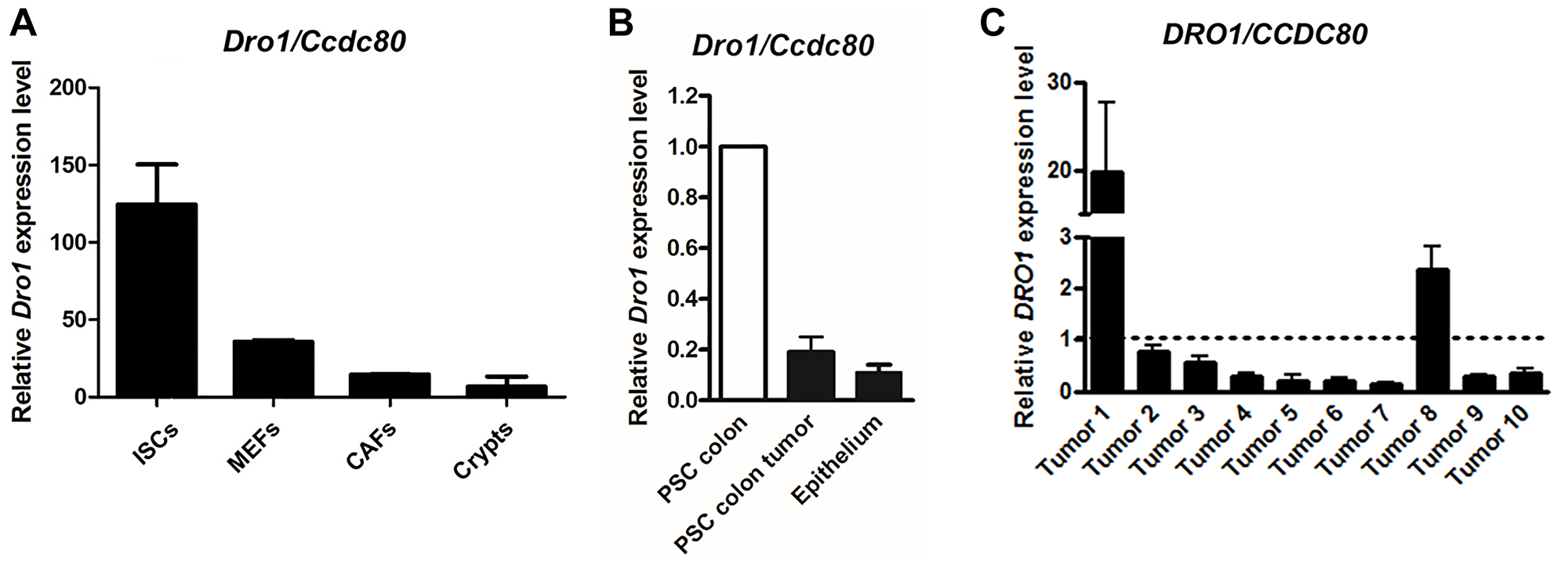
Figure 4: DRO1/CCDC80 is down-regulated in the stromal tumor compartment. (A) Relative Dro1/Ccdc80 mRNA expression in C57BL/6 mouse small intestinal stromal cells (ISCs), mouse embryonic fibroblasts (MEFs; day 18 p.c.), mouse gastric cancer associated fibroblasts (CAFs), and mouse small intestinal crypts (Crypts). (B) Relative Dro1/Ccdc80 mRNA expression in primary stromal cells generated from tumor-free colon from 5-week-old ApcMin/+ mice and from colon tumors from moribund ApcMin/+ mice and in scratched colon epithelium from 5-week-old ApcMin/+ mice. DRO1/CCDC80 expression in PSC from colon tumor and from epithelium is represented relative to expression in normal PSC (set to 1). (C) Relative DRO1/CCDC80 mRNA expression in microdissected human primary tumor stroma from colorectal carcinoma specimens compared to microdissected normal colorectal connective tissue. Matched pairs of tumor stroma and normal adjacent colorectal stroma from 10 patients were analyzed. DRO1/CCDC80 expression in colorectal carcinoma stroma is represented relative to expression in normal stroma (set to 1, see dotted line). Error bars represent standard deviations.
The Christian Research Team concluded in their Oncotarget Research Output, "we identify Dro1/Ccdc80 as tumor suppressor in the tumor microenvironment. DRO1/CCDC80 in the stromal compartment strongly inhibited tumor growth, facilitated apoptosis in cancer cells, and reduced epithelial cell migration. Moreover, stromal DRO1/CCDC80 restrained the formation of epithelial organoids, indicating a possible role for Dro1/Ccdc80 in stemness. Our study provides new insights into the complex interaction between epithelial cells and their microenvironment and contributes to the understanding of cancer development. Future studies are needed to further elucidate the molecular mechanisms underlying Dro1/Ccdc80's microenvironmental tumor suppressive function and to better characterize its role in human cancer.""
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28084
Full text - https://www.oncotarget.com/article/28084/text/
Correspondence to - Jessica I. Christian - [email protected]
Keywords - DRO1, CCDC80, colorectal cancer, tumor microenvironment, tumor suppressor
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105





