Oncotarget Volume 11 Issue 15 reported that since mTOR pathway regulation involves multiple feedback mechanisms that may be differentially activated depending on the degree of mTOR inhibition, we investigated whether rapamycin dosing could be adjusted to achieve chemopreventive efficacy without side effects.
While both doses tested were equally effective in suppressing the proliferation of prostate epithelial cells, a higher dose resulted in the activation of feedback circuits that reduced the drug's tumor preventive efficacy.
Dr. Marina P. Antoch from the Department of Pharmacology and Therapeutics, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA said, "Prostate cancer (PCa) is the second-leading cause of death from cancer in men."
"Prostate cancer (PCa) is the second-leading cause of death from cancer in men."
- Dr. Marina P. Antoch, Department of Pharmacology and Therapeutics, Roswell Park Comprehensive Cancer Center
The phosphatidylinositol 3-kinase /Akt/ mTOR pathway plays a critical role in the development of PCa and progression to castration-resistant PCa: components of the PI3K/Akt/mTOR pathway are altered in 42% of primary and 100% of metastatic PCa cases.
Using a model in which mice with prostate-specific deletion of Pten spontaneously develop PCa, they demonstrated that chronic oral administration of Rapatar at these two doses, in fact, suppresses tumorigenesis.
Histological analysis of prostate tissue showed that Rapatar treatment resulted in reduced proliferation of prostate epithelial cells at both doses tested.
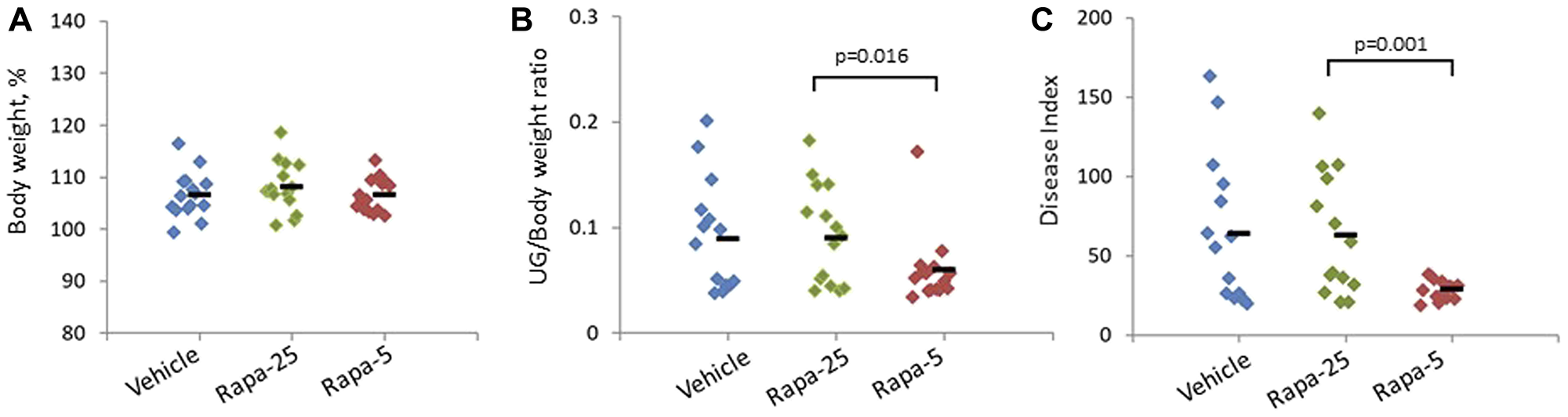
Figure 1: Effect of different Rapatar doses on body weight and PCa tumorigenesis in the psPten–/– mouse model. aOnly tumor types with at least 4 patients are included. bPatients for whom clinical benefit was non-evaluable are included under "no clinical benefit" as follows: ACC n = 1; Colorectal cancer n = 2; Gastrointestinal stromal tumor n = 3; HNSCC n = 3; NSCLC adenocarcinoma n = 1; ovarian n = 3; thymus n = 3. Abbreviations: ACC, adenoid cystic carcinoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; PR, partial response; SD, stable disease.
Further evaluation of the overall severity of prostate tumor development as a combination of multiple parameters demonstrated that a better level of protection was achieved by the low dose of Rapatar.
Overall, the authors' data support an unexpected and simple solution for improving PCa prevention: partial inhibition of mTOR by low doses of Rapatar.
The Antoch Research Team concluded in their Oncotarget Research Article, "we have demonstrated that a novel formulation of rapamycin (Rapatar) can achieve chemopreventive efficacy at low doses that avoid undesirable side effects. This supports the possibility of reviving clinical development of mTOR inhibitor-based approaches for cancer prevention with particular focus on the importance of drug dose."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27550
Full text - https://www.oncotarget.com/article/27550/text/
Correspondence to - Marina P. Antoch - [email protected]
Keywords - prostate cancer, rapamycin, prevention, mTOR, PTEN
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105