Oncotarget published "The potential of PIVKA-II as a treatment response biomarker in hepatocellular carcinoma: a prospective United Kingdom cohort study" which reported that prothrombin induced by vitamin K absence II has recently been validated internationally as a diagnostic biomarker for hepatocellular carcinoma, as part of the GALAD model. However, its role as a treatment response biomarker has been less well explored.
Therefore, these authors undertook a prospective study at a tertiary centre in the UK to evaluate the role of PIVKA-II as a treatment response biomarker in patients with early, intermediate and advanced stage HCC.
In a cohort of 141 patients, they found that PIVKA-II levels tracked concordantly with treatment response in the majority of patients, across a range of different treatment modalities.
Their results demonstrate the potential utility of PIVKA-II as a treatment response biomarker and in predicting microvascular invasion, in a Western population.
Further larger prospective studies are recommended to evaluate PIVKA-II as a treatment response biomarker, within the GALAD model.
Dr. Yuk Ting Ma from The University of Birmingham as well as The University Hospitals Birmingham NHS Foundation Trust said, "Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third most common cause of cancer-related death worldwide."
Hepatocellular carcinoma (HCC) is the seventh most common cancer and the third most common cause of cancer-related death worldwide
Current Clinical Practice guidelines recommend HCC surveillance in high risk individuals, to increase the detection of early-stage HCC, increase curative treatments and thus improve survival.
However, one of the major limitations of HCC surveillance is the lack of reliable serum biomarkers.
Alpha-fetoprotein is the most widely used biomarker for HCC worldwide but, due to its suboptimal sensitivity and specificity in surveillance, the previous American Association for the Study of Liver Diseases and the current European Association for the Study of the Liver guidelines for surveillance recommend six-monthly ultrasound imaging alone.
Prothrombin induced by vitamin K absence II, also known as des-gamma-carboxyprothrombin, is another serum biomarker of HCC that was first reported in 1984.
It has been extensively studied as a diagnostic biomarker and subsequent meta-analyses have confirmed it to have a similar sensitivity to AFP for detecting small HCC.
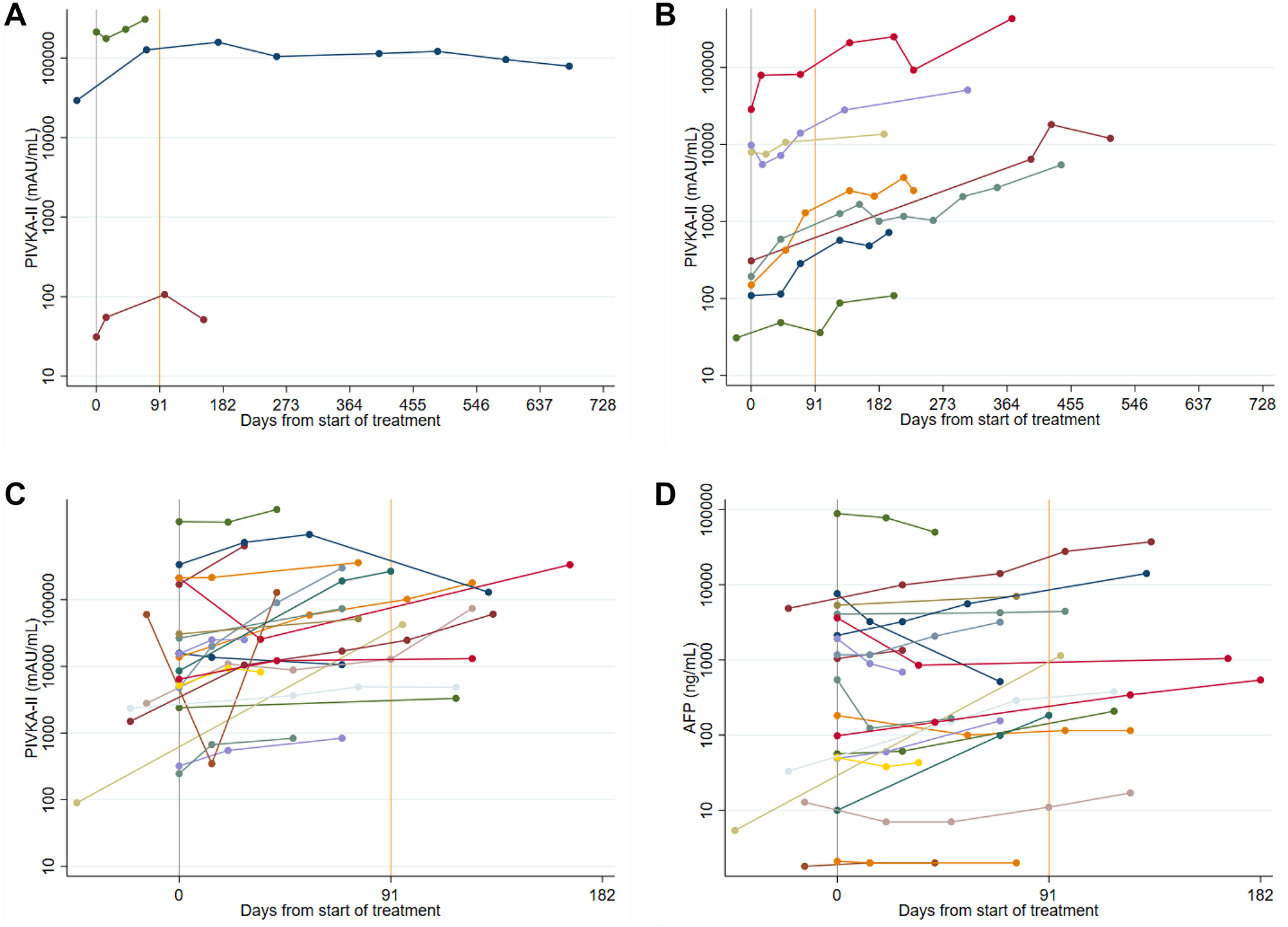
Figure 5: (A) Spider plots of longitudinal PIVKA-II levels in Sorafenib patients: stable disease. A log10 scale has been used for the PIVKA-II values. (B) Spider plots of longitudinal PIVKA-II levels in Sorafenib patients: stable disease followed by progression. A log10 scale has been used for the PIVKA-II values. (C and D) Spider plots of longitudinal PIVKA-II and AFP levels in Sorafenib patients: progressive disease. A log10 scale has been used for the PIVKA-II and AFP values.
The Ting Ma Research Team concluded in their Oncotarget Research Output that microvascular invasion is known to be a major predictor of tumour recurrence after curative liver resection or orthotopic liver transplantation.
In vitro, PIVKA-II has been shown to induce over-expression of epithelial growth factor receptor and vascular endothelial growth factor, directly stimulating the proliferation and migration of vascular endothelial cells.
These authors observed that higher pre-transplant levels of serum PIVKA-II predicted for microvascular invasion, which is consistent with the results from previous studies from Asia and a recent cohort from France.
Collectively, the data thus confirms the value of pre-surgical levels of PIVKA-II in predicting microvascular invasion, again irrespective of disease aetiology.
Future prospective studies are now needed to assess the role of pre-operative levels of PIVKA-II in selecting patients for surgical treatment and/or selecting patients for adjuvant therapy, in addition to its utility in switching between therapies.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28136
Correspondence to - Yuk Ting Ma - [email protected]
Keywords - hepatocellular carcinoma, biomarker, PIVKA-II
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


