Oncotarget published "P10s-PADRE vaccine combined with neoadjuvant chemotherapy in ER-positive breast cancer patients induces humoral and cellular immune responses" which reported that the current study was performed to examine the feasibility, safety and immunogenicity of adding P10s-PADRE to standard-of-care chemotherapy in HR+/HER2− early-stage breast cancer patients.
A significant change in CD16, NKp46 and CD94 expression levels on NK cells and a rise in serum content of IFN- was observed after treatment. The combination therapy is safe and immunogenic with treatment schedule C being immunologically promising.
Dr.Behjatolah Monzavi-Karbassi from The University of Arkansas for Medical Sciences said, "HR+/HER2− breast cancer remains the most common form of breast cancer in the United States."
The positive prognostic effect of ER+ status is limited to 5 years after diagnosis, as the prognosis of HR+ patients becomes worse than that of triple-negative or HER2+ patients among those alive after 5 years since diagnosis. Apparently, the current paradigm of endocrine therapy with or without chemotherapy has not been effective for a considerable number of these patients. Neoadjuvant chemotherapy has become a standard practice for treatment of high-risk localized breast cancer, and pathological complete response to NAC has been a trusted surrogate to assess long-term survival benefits.
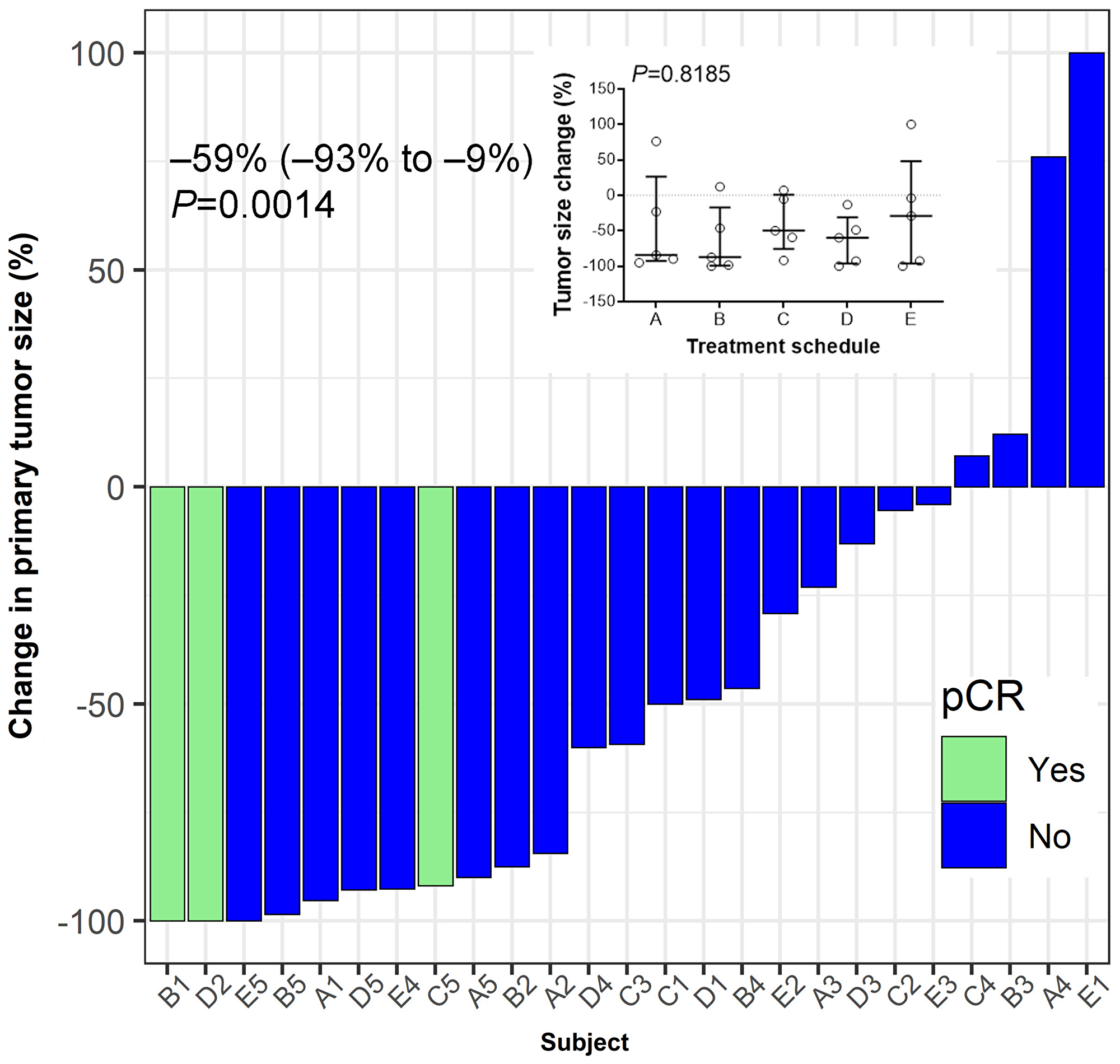
Figure 5: Tumor response to treatment. Hormone-receptor positive breast cancer patients were vaccinated for three consecutive weeks after enrolment in a neo-adjuvant setting. Pathological complete response (pCR) was defined as ypT0/Tis ypN0. Median (IQR, interquartile range) value of the percent change in the post-treatment tumor size from baseline at the time of surgery is shown. Wilcoxon's signed-rank test was used to examine the statistical significance of tumor size reduction (P = 0.0014). Inset summarizes percent tumor-size change based on treatment schedule. No significant differences between schedules are observed (P = 0.8185). Bars indicate median and interquartile range.
In another study, the increase in stromal TILs in residual disease in a patient population, 79% of which had HR+ disease, was associated with improved recurrence-free survival. Therefore, a rational combination therapy that enhances the immune-stimulatory properties of NAC, can provide long-term survival benefits for this patient population. The immunization of breast cancer patients with P10s-PADRE proved feasible, tolerable and immunologically efficacious. After more than 8 years of follow-up, 4 out of 6 vaccinated subjects from the Phase I clinical trial are still alive, with 3 of them in stable condition and 1 subject still in remission.
Moreover, these authors observed that P10s-PADRE-induced immune serum triggered chemosensitivity to paclitaxel in the HR+ZR-75-1 cell line, which suggests that combining the P10s-PADRE vaccine with chemotherapy regimens that include taxanes may prove beneficial in treating breast cancer patients.
Combining the P10s-PADRE vaccine with chemotherapy regimens that include taxanes may prove beneficial in treating breast cancer patients
The Monzavi-Karbassi Research Team concluded in their Oncotarget Research Output, "we demonstrated that treatment schedules have different effect on the numbers of lymphocytes in the tumor microenvironment with schedule C showing higher increase in TILs in residual tumors after treatment. Promoting TILs may positively affect therapeutic modalities, improving long-term survival outcomes [10, 20]. The presence of TILs in residual disease after NAC was associated with better metastasis-free and overall survival [11]. Contrary to our expectation, we did not observe infiltration of NK cells. Instead, the data suggest an increase in stromal CD3+ T cells in residual tumors after treatment. The attraction of T cells into the tumor environment is consistent with our preclinical data showing T-cell-dependent DTH response and a role for T cells in tumor shrinkage using P10-KLH vaccine [30]. Whether more TILs can contribute to better survival outcomes needs to be addressed in future studies. Lack of enough tissues prohibited identification of T-cell subsets that is a limitation of our study."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28083
Full text - https://www.oncotarget.com/article/28083/text/
Correspondence to - Behjatolah Monzavi-Karbassi - [email protected]
Keywords - cancer vaccine, peptide mimotopes, combination therapy, breast cancer
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



