Volume 11, Number 18 of @Oncotarget reported that the authors previously reported that nutlin-3 induced PARP1 degradation in a p53-dependent manner in mouse fibroblasts, suggesting nutlin-3a may be a PARP1 suppressor.
Short-term nutlin-3a treatment elevated the levels of PARylated PARP1, suggesting nutlin-3a promoted PARylation of PARP1, thereby inducing its proteasomal degradation.
This study revealed that nutlin-3a is a PARP1 suppressor that induces PARP1 proteasomal degradation by binding to MDM2 and promoting auto PARylation of PARP1.
Dr. Masaki Kobayashi and Dr. Yoshikazu Higami from The Laboratory of Molecular Pathology & Metabolic Disease, Faculty of Pharmaceutical Sciences as well was The Translational Research Center, Research Institute of Science and Technology at The Tokyo University of Science in Noda Chiba Japan said, "The poly (ADP-ribose) polymerase (PARP) family consists of 17 enzymes and functions in several cellular processes including DNA recombination and repair, cellular proliferation, apoptosis in ischemic conditions and necrotic cell death."
"The poly (ADP-ribose) polymerase (PARP) family consists of 17 enzymes and functions in several cellular processes including DNA recombination and repair, cellular proliferation, apoptosis in ischemic conditions and necrotic cell death."
- Dr. Masaki Kobayashi and Dr. Yoshikazu Higami, The Laboratory of Molecular Pathology & Metabolic Disease, Faculty of Pharmaceutical Sciences & Translational Research Center, Research Institute of Science and Technology at The Tokyo University of Science
PARP1 is activated by DNA damage caused by free radicals or other agents and plays an important role in single-strand DNA repair through the base excision pathway.
Activated PARP1 PARylates and recruits DNA repair factors such as X-ray repair cross-complementing gene 1, DNA ligase III, and polynucleotide kinase to damaged sites, resulting in DNA repair.
Furthermore, PARP1 is regulated by ubiquitination-mediated proteasomal degradation, and the ubiquitination of PARP1 has been shown to be dependent on PARylation by PARP1 itself, in other words via auto PARylation.
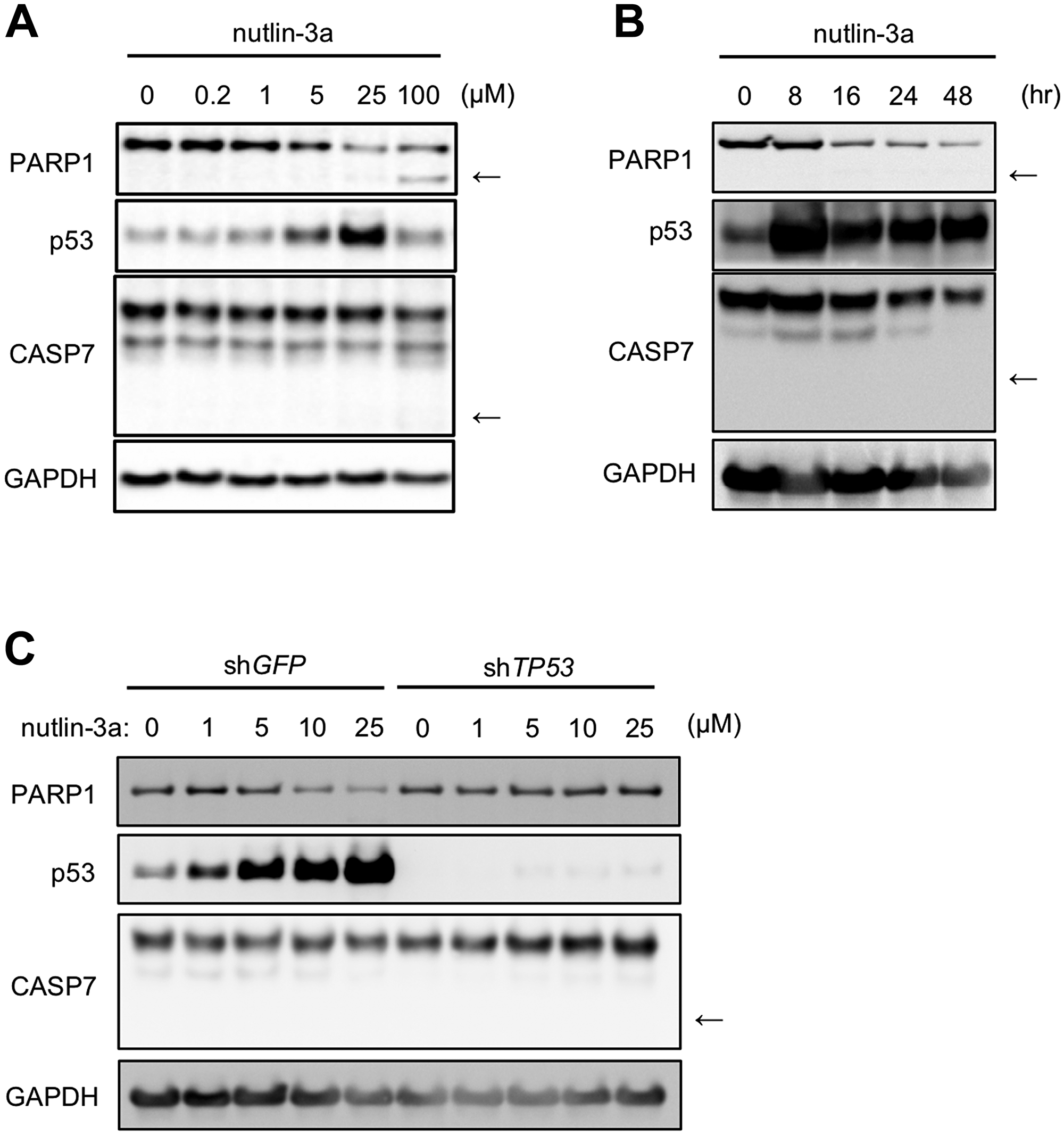
Figure 1: Nutlin-3a reduces PARP1 protein levels in MCF-7 cells, a human breast cancer cell line. (A) MCF-7 cells were treated with indicated concentrations of nutlin-3a for 24 h. (B) MCF-7 cells were treated with 10 μM nutlin-3a for the indicated times. (C) MCF-7/shGFP and MCF-7/shTP53 cells were treated with indicated concentrations of nutlin-3a for 24 h. The cell lysates were analyzed by immunoblotting using the indicated antibodies. In the PARP1 and CASP7 panels, arrows indicate apoptotic fragments. GAPDH was used as a loading control.
For example, ring finger protein 146 induces poly-ubiquitination and degradation of auto PARylated PARP1, regulating the DNA damage response.
Likewise, checkpoint with forkhead and ring finger domains preferentially interacts with auto PARylated PARP1 under mitotic stress conditions and then induces PARP1 degradation via the ubiquitin-proteasome system.
PARP1 inhibitors are expected to serve as therapeutic agents for various cancers such as breast cancer susceptibility gene 1 - and BRCA2-associated hereditary breast and ovarian cancers, prostate cancer, non-small cell lung cancer, and Ewing sarcoma.
The Kobayashi/Higami Research Team concluded in their Oncotarget Research Article, "Our previous and present studies further demonstrate that nutlin-3a is a PARP1 suppressor with the ability to promote degradation of PARP1 protein."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27581
Full text - https://www.oncotarget.com/article/27581/text/
Correspondence to - Masaki Kobayashi - [email protected] and Yoshikazu Higami - [email protected]
Keywords - nutlin-3a, PARP1, breast cancer, proteasomal degradation, autoPARylation
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


