Oncotarget published "Mutational profile of skin lesions in hepatocellular carcinoma patients under tyrosine kinase inhibition: a repercussion of a wide-spectrum activity" which reported that SL were prospectively collected in 311 HCC patients who started sorafenib.
SL from sorafenib cohort were compared to those from a control patient group selected to match SL type and demographics.
HRAS, KRAS and BRAF mutations were analyzed by CAST-PCR, mutated p53 and MAPK pathway activation by immunohistochemistry and immune infiltration by hematoxylin-eosin staining. Eighty-eight out of 311 patients developed DAE and 7.4% SL required histological assessment.
HRAS and KRAS mutations were detected in 4 SL, while no mutations showed in control SL.
The onset of SL and their molecular profile did not impact negatively on patient's prognosis, but intense proliferation of SL may reflect compensatory activation of MAPK pathway and warrants their close monitoring.
Dr. Loreto Boix and Dr. Alba Díaz from The Universitat de Barcelona said, "Sorafenib is an oral multitarget tyrosine kinase inhibitor directed to vascular endothelial growth factor receptor (VEGFR)-1, -2, -3, platelet-derived growth factor receptor (PDGFR)- b, c-KIT, RET, FLT-3 and BRAF."
"Sorafenib is an oral multitarget tyrosine kinase inhibitor directed to vascular endothelial growth factor receptor (VEGFR)-1, -2, -3, platelet-derived growth factor receptor (PDGFR)- b, c-KIT, RET, FLT-3 and BRAF."
This agent demonstrated survival benefit in patients with hepatocellular carcinoma and represents one of the first line treatments for HCC patients.
Besides HCC, sorafenib is also indicated for advanced renal cell carcinoma, locally recurrent or metastatic radioactive iodine-refractory differentiated thyroid carcinoma and refractory desmoid tumors.
Since the approval of sorafenib, case reports and small series have described the onset of proliferative and inflammatory skin lesions in patients undergoing treatment, such as squamous cell carcinoma, keratoacanthoma, actinic keratosis, cystic folliculitis and basal cell carcinoma.
These lesions were predominantly reported in patients with renal cell carcinoma, while anecdotal cases have been described in HCC.
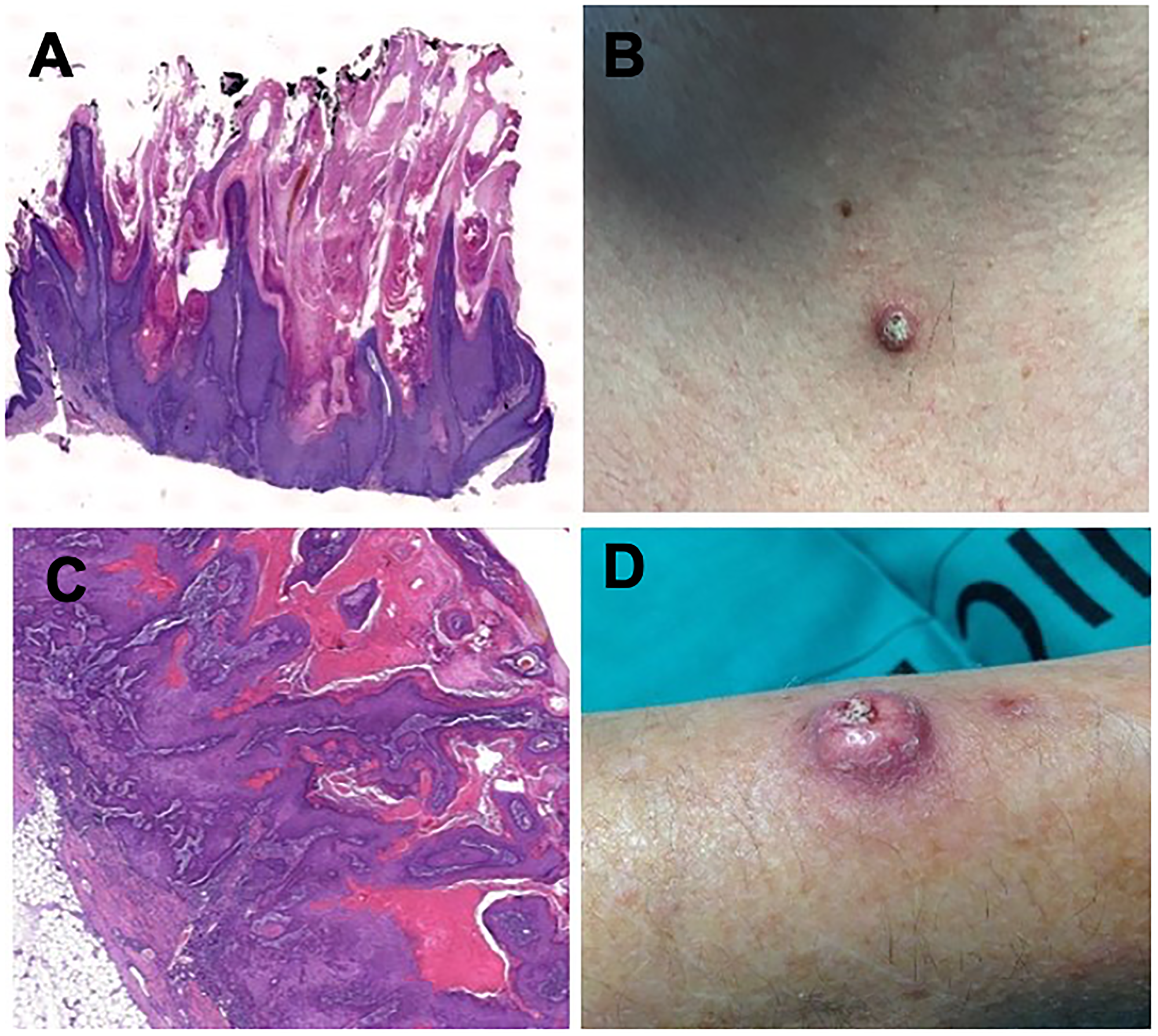
Figure 2: Histologic findings in hematoxylin and eosin staining 2x magnification (A) and clinical aspect (B) of a keratoacanthoma in a patient under sorafenib treatment. Histologic findings in hematoxylin and eosin staining 2x magnification (C) and clinical aspect (D) of a squamous-cell carcinoma in a patient under sorafenib treatment.
In the present study, these authors aimed to describe clinicopathological and molecular features of SL requiring excisional procedures, in a prospective cohort of patients under sorafenib treatment for HCC.
The Boix/Díaz Research Team concluded in their Oncotarget Research Output that this study is limited by the low number of patients with each type of SL and to the restricted set of mutations analyzed.
This prevents us from drawing a complete causative chain between sorafenib and molecular alterations driving to SL.
The authors expect these data may prime other groups to explore skin lesions under cancer therapy and increase the knowledge in this field.
The underlying molecular events and the influence of the immune system should be further explored in order to provide useful information on the impact on these events in the clinical course and outcomes of patients with HCC under any other treatment besides sorafenib.
From a clinical point of view, it is important to pay attention to the need to closely monitoring patients in order to detect newly developed SL during treatment.
DOI - https://doi.org/10.18632/oncotarget.27891
Full text - https://www.oncotarget.com/article/27891/text/
Correspondence to - Loreto Boix - [email protected] and Alba Díaz - [email protected]
Keywords - hepatocellular carcinoma, tyrosine kinase inhibitors, treatment adverse events, skin lesions, molecular profiling
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105