The cover for issue 30 of Oncotarget features Figure 4, "RNAseq results demonstrating differences between normal, cancer, and redirected cells," by Frank-Kamenetskii, et al. which reported that the influence of breast cancer cells on normal cells of the microenvironment, such as fibroblasts and macrophages, has been heavily studied but the influence of normal epithelial cells on breast cancer cells has not.
Here using in vivo and in vitro models the Oncotarget authors demonstrate the impact epithelial cells and the mammary microenvironment can exert on breast cancer cells.
Under specific conditions, signals that originate in epithelial cells can induce phenotypic and genotypic changes in cancer cells.
The authors have termed this phenomenon “cancer cell redirection.” Once breast cancer cells are redirected, either in vivo or in vitro, they lose their tumor forming capacity and undergo a genetic expression profile shift away from one that supports a cancer profile towards one that supports a non-tumorigenic epithelial profile.
These findings indicate that epithelial cells and the normal microenvironment influence breast cancer cells and that under certain circumstances restrict proliferation of tumorigenic cells.
Dr. Brian W. Booth from Clemson University said, "Tissue microenvironments are complex regions that consist of multiple cell types such as epithelial cells, adipocytes, fibroblasts, vascular endothelial cells, resident and transient immune cells, and somatic stem cells"
"Tissue microenvironments are complex regions that consist of multiple cell types such as epithelial cells, adipocytes, fibroblasts, vascular endothelial cells, resident and transient immune cells, and somatic stem cells"
Tissue microenvironments are complex regions that consist of multiple cell types such as epithelial cells, adipocytes, fibroblasts, vascular endothelial cells, resident and transient immune cells, and somatic stem cells.
Using rodent models, it was discovered that when mammary epithelial cells were transplanted into a mammary fat pads of pre-pubescent female mice devoid of endogenous epithelium an entire functional mammary outgrowth could be recapitulated regardless of age or parity status of the transplanted cells.
When dispersed cell suspensions of mammary epithelial cells are used in these models the cells participate in the formation of new microenvironments allowing for the normal development of mammary outgrowths.
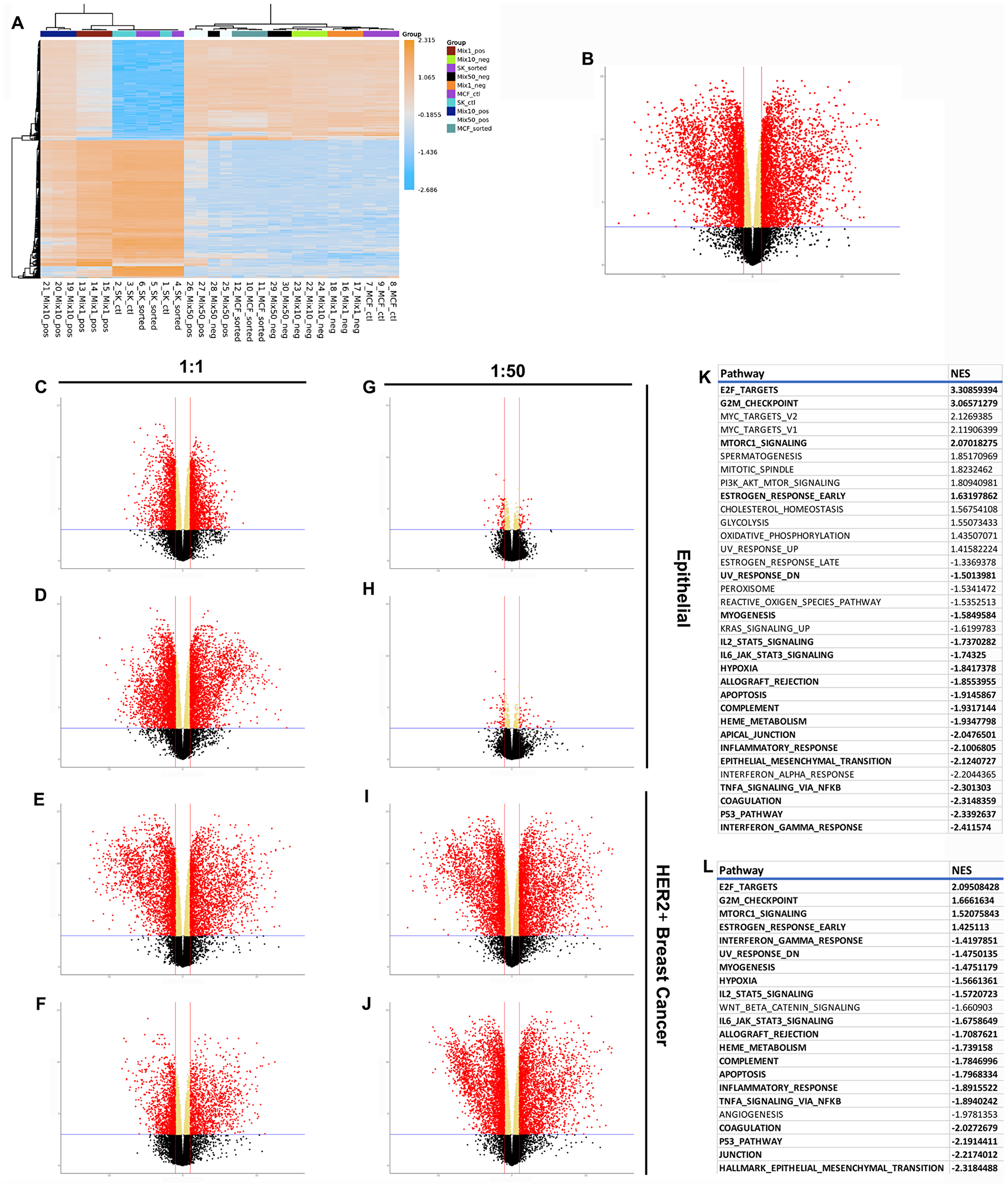
Figure 4: RNAseq results demonstrating differences between normal, cancer, and redirected cells. (A) Clustered heatmap of the 500 most variable genes, by expression, across all samples. Volcano plots show differential expression of 15647 measured genes contrasting (B) cancer and epithelial cells, (C) 1:1 HER2- fraction and epithelial cells, (D) 1:1 HER2+ fraction and epithelial cells, (E) 1:1 HER2- fraction and breast cancer cells, (F) 1:1 HER2+ fraction and breast cancer cells, (G) 1:50 HER2- fraction and epithelial cells, (H) 1:50 HER2+ fraction and epithelial cells, (I) 1:50 HER2- fraction and breast cancer cells, (J) 1:50 HER2+ fraction and breast cancer cells. Dashed blue horizontal lines are the adjusted p-value threshold (≤ 0.05); dashed red lines are fold change thresholds (fold change ≥ 2.0 or ≤-2.0). Red dots are genes that passed both thresholds and are those reported as DE in this study. (K) Table of significantly de/activeated pathways between breast cancer cells and epithelial cells. (L) Table of significantly de/activated pathways between breast cancer cells and redirected cells. Pathways highlighted in bold appear in both Tables.
Stem cells isolated from the central nervous system, bone marrow, testes, and embryonic stem cells have been introduced into reforming mammary microenvironments and adopted mammary epithelial phenotypes.
Lineage-traced daughter cells of the non-mammary stem cells participated in the normal development of mammary ductal trees and differentiated into luminal epithelial cells, myoepithelial cells, and milk protein-producing secretory epithelial cells during lactation.
The Booth Research Team concluded in their Oncotarget Research Paper, "our data collectively argue that epithelial cells provide signals that influence HER2+ breast cancer cells to reduce tumor formation. The reduction in tumor-forming capacity is due to a shift in gene expression profiles from a tumorigenic towards a normal epithelial profile. The phenotypic switch, known as cancer cell redirection, includes changes in the activity of multiple intracellular signaling pathways. Modulation of these affected pathways may be a new approach towards cancer treatments."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27679
Full text - https://www.oncotarget.com/article/27679/text/
Correspondence to - Brian W. Booth - [email protected]
Keywords - breast cancer, cancer cell redirection, microenvironment, stem cells
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



