Oncotarget published "Impact factor and citation metrics in phase III cancer trials" which reported that a journal impact factor is often used to measure research quality and importance. The authors assessed trial factors associated with the publication of cancer trials in journals with higher IF and publications receiving higher citations. Seven-hundred ninety manuscripts were included in their study.
Trials that met their primary endpoint were more commonly published in journals with higher IF. Lastly, publications of trials leading to FDA approvals, and publications of trials in journals with higher IF were associated with increased RCR.
Trials that met their primary endpoint were more commonly published in journals with higher IF
Dr. Cullen M. Taniguchi and Dr. Ethan B. Ludmir both from The University of Texas MD Anderson Cancer Center said, "Clinical trial reporting remains the cornerstone of disseminating clinical cancer research."
The journal that ultimately publishes trial results plays a key role in the dissemination of scientific information. Generally, trialists and sponsoring bodies aim to publish trial results to the widest possible audience, which often involves aiming for publication in journals with a higher impact factor. Thus, the selection of manuscripts published in journals with high IF is based on a wide array of factors, including study impact, novelty, design, and quality.
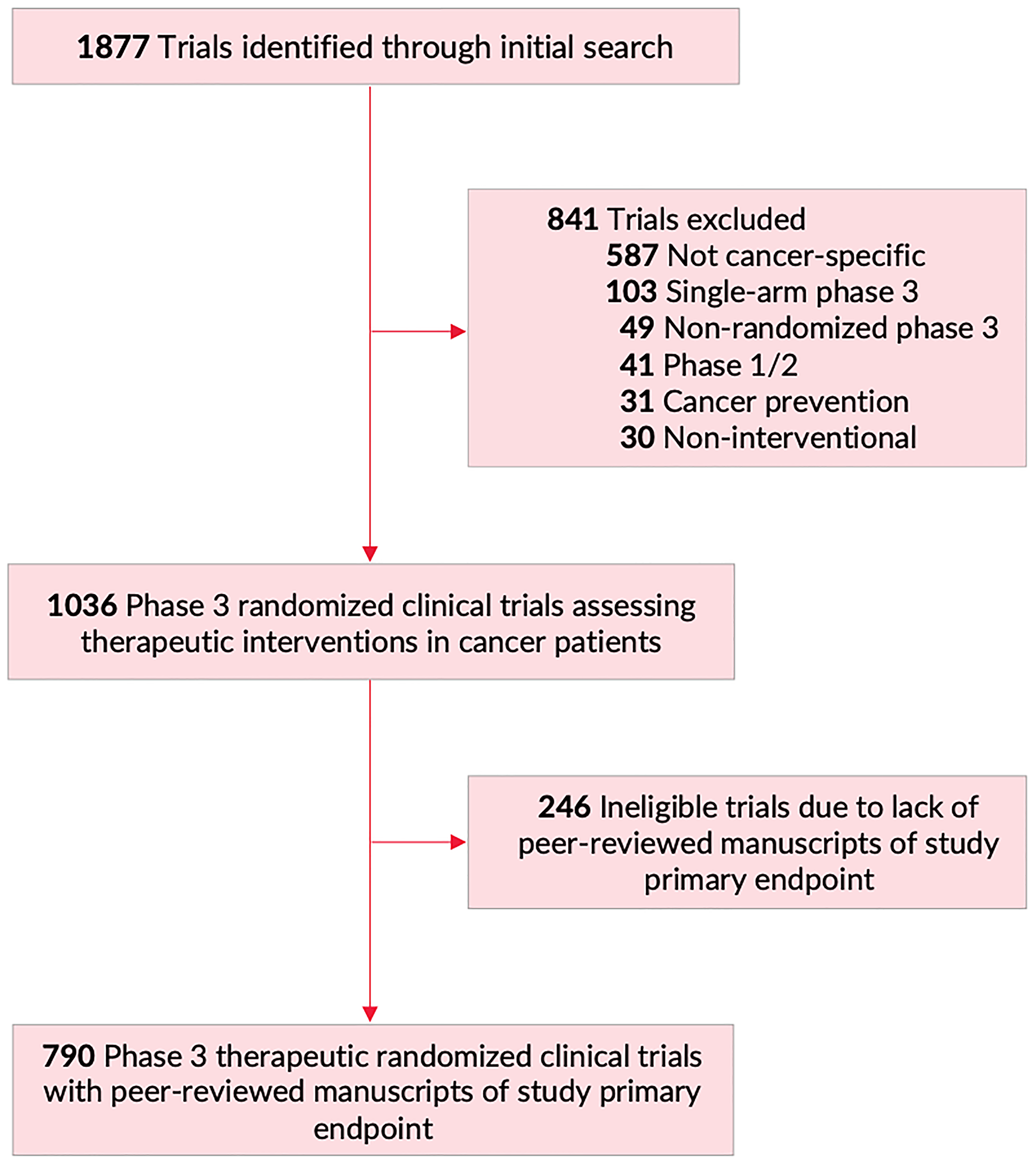
Figure 1: Flowchart of trial screening and inclusion. Of 1877 trials identified on https://clinicaltrials.gov/ (February 20, 2020), 841 were excluded, for a final total of 1036 phase III randomized clinical trials assessing therapeutic interventions in patients with cancer. Of those, 790 trials had peer-reviewed manuscripts of primary study endpoint, and were included in primary analysis for this manuscript.
Furthermore, owing to the nature of IF being highly based on citations, many alternate factors could play a role in selecting which trial reports to publish in high-impact journals. In this study, the authors investigated factors associated with the publication of trial results in journals with higher IF.
The Baer Research Team concluded in their Oncotarget Research Output, "trials meeting their primary endpoints and trials leading to subsequent FDA drug approvals are often published in journals with a higher IF. Analyzing those factors associated with differential publication of oncologic trial results may help in understanding the complex role of bibliometrics in publishing cancer trials."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28044
Full text - https://www.oncotarget.com/article/28044/text/
Correspondence to - Cullen M. Taniguchi - [email protected] and Ethan B. Ludmir - [email protected]
Keywords - oncology, journal impact factor, clinical trials, FDA, industry
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105