Oncotarget published "Hepatitis B x antigen (HBx) is an important therapeutic target in the pathogenesis of hepatocellular carcinoma" which reported that despite the availability of highly efficacious vaccines, universal screening of the blood supply for virus, and potent direct acting anti-viral drugs, there are more than 250 million carriers of HBV who are at risk for the sequential development of hepatitis, fibrosis, cirrhosis and hepatocellular carcinoma.
More than 800,000 deaths per year are attributed to chronic hepatitis B. Many different therapeutic approaches have been developed to block virus replication, and although effective, none are curative.
Although given little attention, HBx is an important therapeutic target because it contributes importantly to HBV replication, in protecting infected cells from immune mediated destruction during chronic infection, and in the development of HCC.
Thus, the development of therapies targeting HBx, combined with other established therapies, will provide a functional cure that will target virus replication and further reduce or eliminate both the morbidity and mortality associated with chronic liver disease and HCC.
Simultaneous targeting of all these characteristics underscores the importance of developing therapies against HBx.
Simultaneous targeting of all these characteristics underscores the importance of developing therapies against HBx.
Dr. Mark A. Feitelson from The Azad University said, "HBV is a major etiologic agent responsible for the development of chronic liver disease (CLD) which may resolve or progress to HCC."
Since the carrier state and progression of CLD are major risk factors for the development of HCC, alternative approaches need to be developed to slow the progression of CLD and reduce the risk of HCC and/or more effectively treat HCC upon diagnosis.
The HBV encoded x antigen, HBx, is the virus contribution to the development of HCC.
Many of the properties that promote CLD progression overlap with hallmarks of cancer, suggesting that HBx contributes to HCC by epigenetically altering patterns of host gene expression, while immune mediated CLD is the host contribution to tumor development.
Accordingly, this perspective piece highlights the role of HBx in virus replication and the pathogenesis of HCC, thereby providing strong rationale for pursuing HBx as a therapeutic target of drug development.
This also provides a very large therapeutic window, extending from the time of infection when HBx promotes virus gene expression and virus replication to the development of HCC decades later.
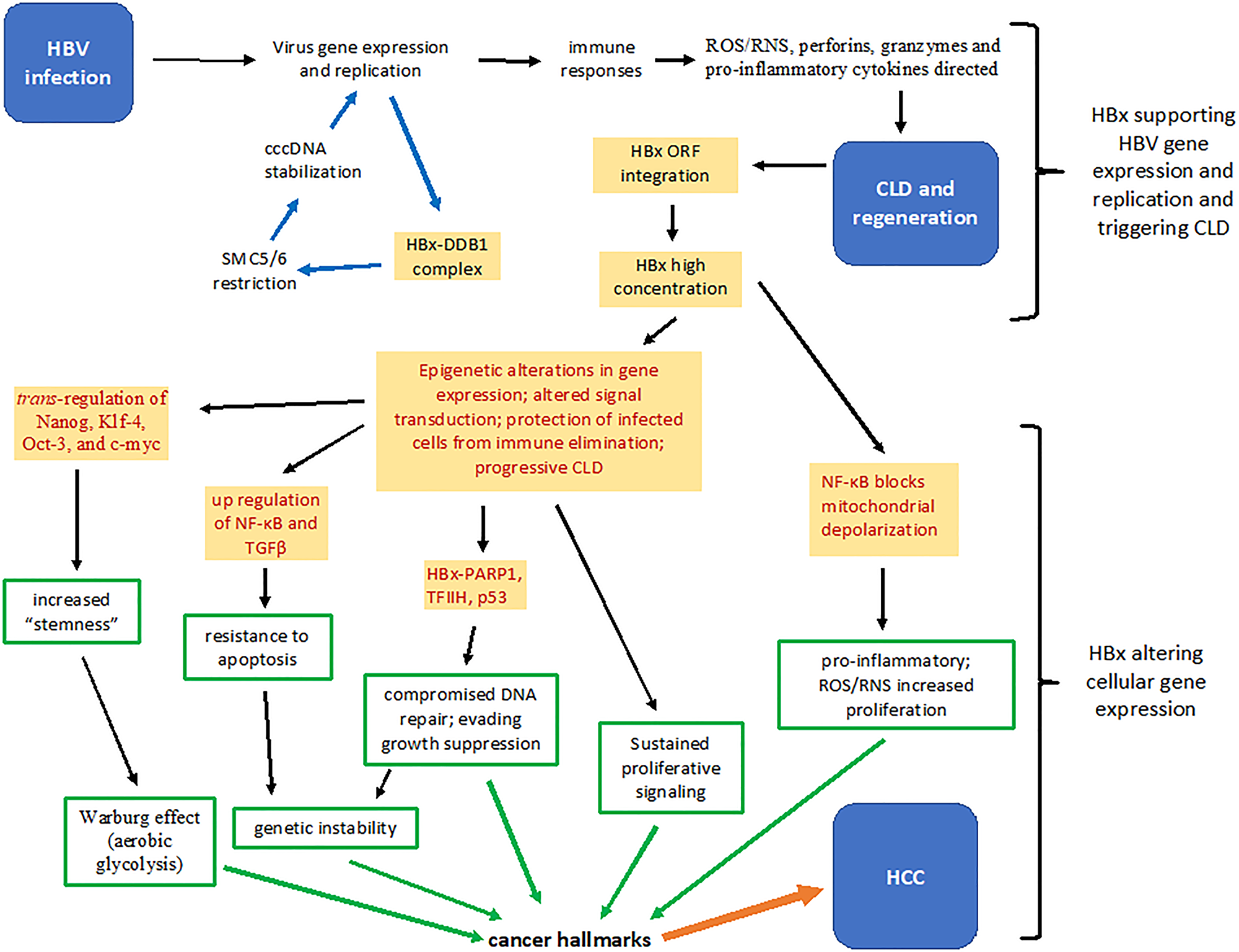
Figure 1: Summary of several HBx functions that contribute to the pathogenesis of chronic liver disease and hepatocellular carcinoma. Upon infection, HBx stimulates virus gene expression and replication by binding a protein complex containing DDB1 and by inactivating the restriction factor SMC5/6 (blue arrows). Hepatocytes replicating virus may trigger immune responses that result in the generation of reactive oxygen and nitrogen species, perforins, granzymes and pro-inflammatory cytokines. Successive bouts of chronic liver disease and hepatocellular regeneration results in increased integration of HBV DNA fragments encoding HBx, which over time accumulates intracellularly. Cytoplasmic and nuclear HBx epigenetically alter patterns of host gene expression that partially protects infected hepatocytes from immune mediated destruction, thereby allowing continued virus replication (red print). Constitutive activation of NF-κB by HBx is both hepatoprotective and pro-inflammatory. HBx promotes the development of “stemness” by up-regulating expression of Nanog, Klf-4, Oct-3 and c-myc. HBx contributes to fibrosis by promoting expression of TGFβ, and inactivates tumor suppressors, such as p53, and inhibits the activity of poly (ADP-ribose) polymerase (PARP1), permitting accumulation of mutations. Mitochondrial associated HBx also alters cellular energetics and the cellular redox state (increases oxidative stress) (red print). Each of these activities contribute to molecular changes that reflect in altered cell behavior (green arrows) that are defined as cancer hallmarks, which in combination, contribute to the development of hepatocellular carcinoma (orange arrow). CLD: chronic liver disease; DDB1: DNA damage-binding protein 1; HBV: hepatitis B virus, ORF: open reading frame; NF-κB: nuclear factor kappa B; RNS: reactive nitrogen species; ROS: reactive oxygen species; TFIIH: transcription factor II H; TGFβ: transforming grow factor beta.
The Feitelson Research Team concluded in their Oncotarget Research Output that accumulating evidence suggests that HBx contributes to virus replication, protects infected hepatocytes against immune elimination, and contributes to multi-step hepatocarcinogenesis.
The fact that HBx alters patterns of gene expression epigenetically, suggests that compounds made in the body or synthetically prepared against targets of the epigenetic machinery altered by HBx would help to lower the risk for the progression of CLD to HCC by re-establishing hepatocellular homeostasis.
While there are promising compounds being developed against HBx, as discussed above, most are against virus replication.
Given that gut bacteria make both pro- and anti-inflammatory metabolites that help to maintain immunological homeostasis via immuno-regulation, treatment resulting in the resolution of dysbiosis may mitigate CLD and its progression to HCC.
This would potentially provide an epigenetic approach for reversing the effects of HBx in the liver and generating a functional cure characterized by little or no virus replication, amelioration of CLD, and either delayed onset or prevention of HCC.
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.28077
Correspondence to - Mark A. Feitelson - [email protected]
Keywords - chronic liver disease, hepatocellular carcinoma, HBx, functional cure, epigenetic
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


