Oncotarget Volume 11, Issue 7 | The Research Team has previously reported that DNA-dependent protein kinase facilitates HIV transcription by interacting with the RNA polymerase II complex recruited at HIV LTR.
DNA-PK inhibition/knockdown leads to the severe impairment of HIV replication and reactivation of latent HIV provirus.
Dr. Mudit Tyagi from the Center for Translational Medicine at Thomas Jefferson University, the Division of Infectious Diseases in the Department of Medicine at George Washington University, as well as the Department of Microbiology, Immunology and Tropical Medicine at George Washington University, said: "Combination anti-retroviral therapy (cART), commonly known as highly active antiretroviral therapy (HAART), is able to suppress replication of human immunodeficiency virus (HIV) quite effectively, oftentimes with a single daily dose."
"Combination anti-retroviral therapy (cART), commonly known as highly active antiretroviral therapy (HAART), is able to suppress replication of human immunodeficiency virus (HIV) quite effectively, oftentimes with a single daily dose."
- Dr. Mudit Tyagi, the Center for Translational Medicine at Thomas Jefferson University, the Division of Infectious Diseases in the Department of Medicine at George Washington University, as well as the Department of Microbiology, Immunology and Tropical Medicine at George Washington University
Phosphorylation of Ser5 residue of the RNAP II CTD is linked to the initiation phase of transcription, whereas phosphorylation of Ser2 is found to be correlated with the elongation phase of transcription, also during the HIV gene expression.
Moreover, the authors have shown that DNA-PK is a component of RNAP II holoenzyme, recruited at HIV LTR, and it rides along RNAP II throughout the HIV genome.
In their investigation, by attenuating the activity or cellular levels of DNA-PK, they have established the role of DNA-PK not only in activating TRIM28 through phosphorylation but also in recruiting TRIM28 and phosphorylated TRIM28 at HIV LTR. In a prior publication, these researchers suggested an important role of DNA-PK during HIV transcription and documented the continuous presence and gliding of DNA-PK with RNAP II along the HIV genome during transcription.
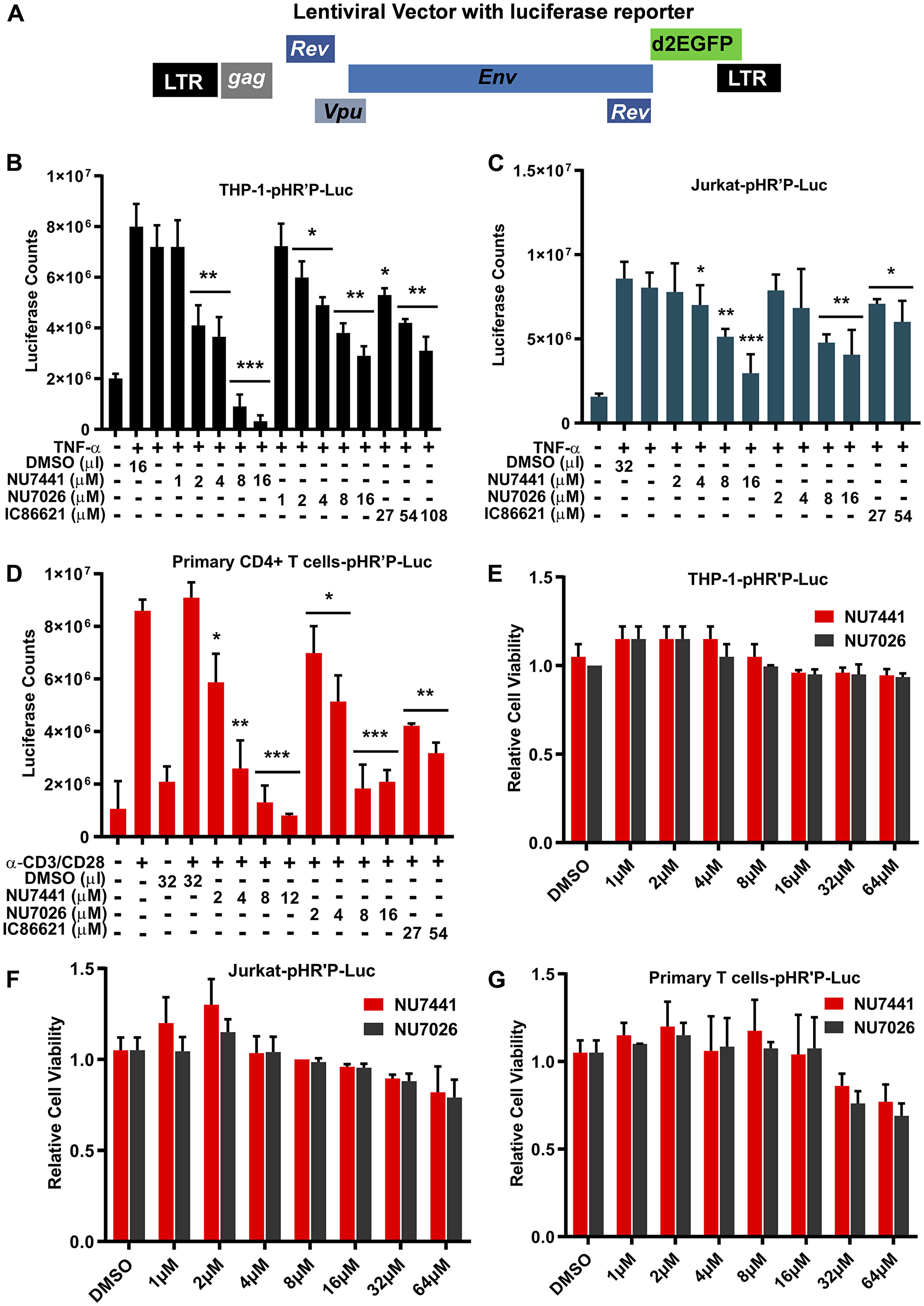
Figure 1: DNA-PK inhibitors repress HIV transcription without showing cytotoxicity. (A) HIV- based lentiviral vector, pHR′P-Luc, which express luciferase reporter gene through HIV LTR promoter. (B) THP-1, a monocytic cell line, (C) Jurkat, a T cell line, and (D) primary CD4+ T cells carrying integrated latent pHR′P-Luc provirus in their genome were treated with indicated amounts of the DNA-PK inhibitors NU7441, NU7026, IC86621 or DMSO solvent-control along with or without 10 ng/ml of TNF-α. After 48 hours cells were lysed, and equal amount of proteins were used for luciferase assays. MTS cell viability assays were also performed after 5 days of culture (E) THP-1-pHR′P-Luc, (F) Jurkat-pHR′P-Luc and (G) primary CD4+ T cells-pHR′P-Luc. The results represent the Mean ± SD of three independent assays. The p value of statistical significance was set at either: p < 0.05 (*), 0.01 (**), 0.001 (***), or 0.0001 (****) when compared with DMSO treated stimulation as control.
They went on to show that DNA-PK promotes the HIV transcriptional initiation by catalyzing the phosphorylation of RNAP II CTD at Ser5; it relieves the pausing of RNAP II by catalyzing the phosphorylation of TRIM28 at S824.
By catalyzing the phosphorylation of TRIM28, specifically at residue S824, DNA-PK converts TRIM28 from a pausing factor to an elongating factor, thus relieving RNAP II pausing.
Additionally, DNA-PK supports the elongation phase of HIV transcription by enhancing the processivity of RNAP II through augmenting the CTD phosphorylation at Ser2, both directly and via facilitating the recruitment of P-TEFb at HIV LTR.
The Tyagi Research Team concluded in their Oncotarget Research Paper that their findings unraveled the molecular mechanisms utilized by DNA-PK to enhance HIV transcription and confirmed the physiological relevance of DNA-PK inhibitors in restricting HIV gene expression, replication, and reactivation of latent provirus.
Sign up for free Altmetric alerts about this article
Full text - https://doi.org/10.18632/oncotarget.27487
Correspondence to - Mudit Tyagi - [email protected]
Keywords - HIV, DNA-PK, transcription, replication, DNA-PK inhibitors
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.impactjournals.com/ or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


