Oncotarget published "Next-generation multimodality of nutrigenomic cancer therapy: sulforaphane in combination with acetazolamide actively target bronchial carcinoid cancer in disabling the PI3K/Akt/mTOR survival pathway and inducing apoptosis" which reported that microscopic imaging, immunocytochemistry, wound healing assay, caspase-cleaved cytokeratin 18 CytoDeath ELISA assay, immunofluorescence labeling assays for apoptosis, hypoxia, Western Blotting, Tunnel assay, measurement of 5-HT secretion by carbon fiber amperometry assay, quantitative methylation-specific PCR, morphologic changes, cell viability, apoptosis activity and the expression levels of phospho-Akt1, Akt1, HIF-1α, PI3K, p21, CAIX, 5-HT, phospho-mTOR, and mTOR in xenografts derived from typical H727 and atypical H720 BC cell lines.
Combining AZ SFN reduced tumor cell survival compared to each agent alone, both in vitro and in vivo xenograft tissues.
AZ SFN targeted multiple pathways involved in cell cycle, serotonin secretion, survival, and growth pathways, highlighting its therapeutic approach.
Both H727 and H720 cells were associated with induction of apoptosis, upregulation of the p21 cell cycle inhibitor, and downregulation of the PI3K/Akt/mTOR pathway, suggesting that the PI3K/Akt/mTOR pathway is a primary target of the AZ SFN combination therapy.
Combining SFN AZ significantly inhibits the PI3K/Akt/mTOR pathway and significantly reducing 5-HT secretion in carcinoid syndrome.
Combining SFN AZ significantly inhibits the PI3K/Akt/mTOR pathway and significantly reducing 5-HT secretion in carcinoid syndrome.
Dr. Herman Yeger from the The Hospital for Sick Children, Dr. Reza Bayat Mokhtari also from The Hospital for Sick Children but as well as The University of Massachusetts, and The Queen's University and Dr. Myron R. Szewczuk also from The Queen's University said, "Malignant cells are characterized by the upregulation and activation of many survival signaling pathways involved in proliferation, apoptosis, invasion, and angiogenesis"
The phosphatidylinositol 3-Kinase-Akt pathway is one of the critical cancer pathogenic pathways with widespread downstream effects involving cell cycle survival and hypoxic metabolic response, angiogenesis, and metastasis.
This pathway regulates the tumor microenvironment and promotes tumor cell survival by reducing reactive oxygen species and subsequent DNA damage within tumor cells.
Also, using an orthotopic lung model of bronchial carcinoid, cell line-derived spheroids, and patient tumor-derived 3rd generation spheroids under supplemental stroma media conditions, the authors reported that SFN in combination with AZ significantly inhibited the growth of the BC cell lines, the formation of spheroids containing a higher fraction of tumor-initiating cells exhibiting a stemness phenotype, and in reducing tumor formation in immunocompromised mice.
In this study, they investigated the mechanism by which SFN combined with AZ exerts its nutrigenetic therapeutic effect on BC cell lines and in BC xenograft tissues derived from H727 and H720 BC cells previously developed in NOD/SCID mice.
The combination of SFN and AZ reduced the pro-survival PI3K/Akt/mTOR pathway, upended pro-survival hypoxia-mediated pathways resulting in decreased 5-HT secretion, migration of H727 and H720 cells in xenografts, and targeted the pro-survival Keap1/Nrf2 pathway, with an overall marked induction of BC cell apoptosis.
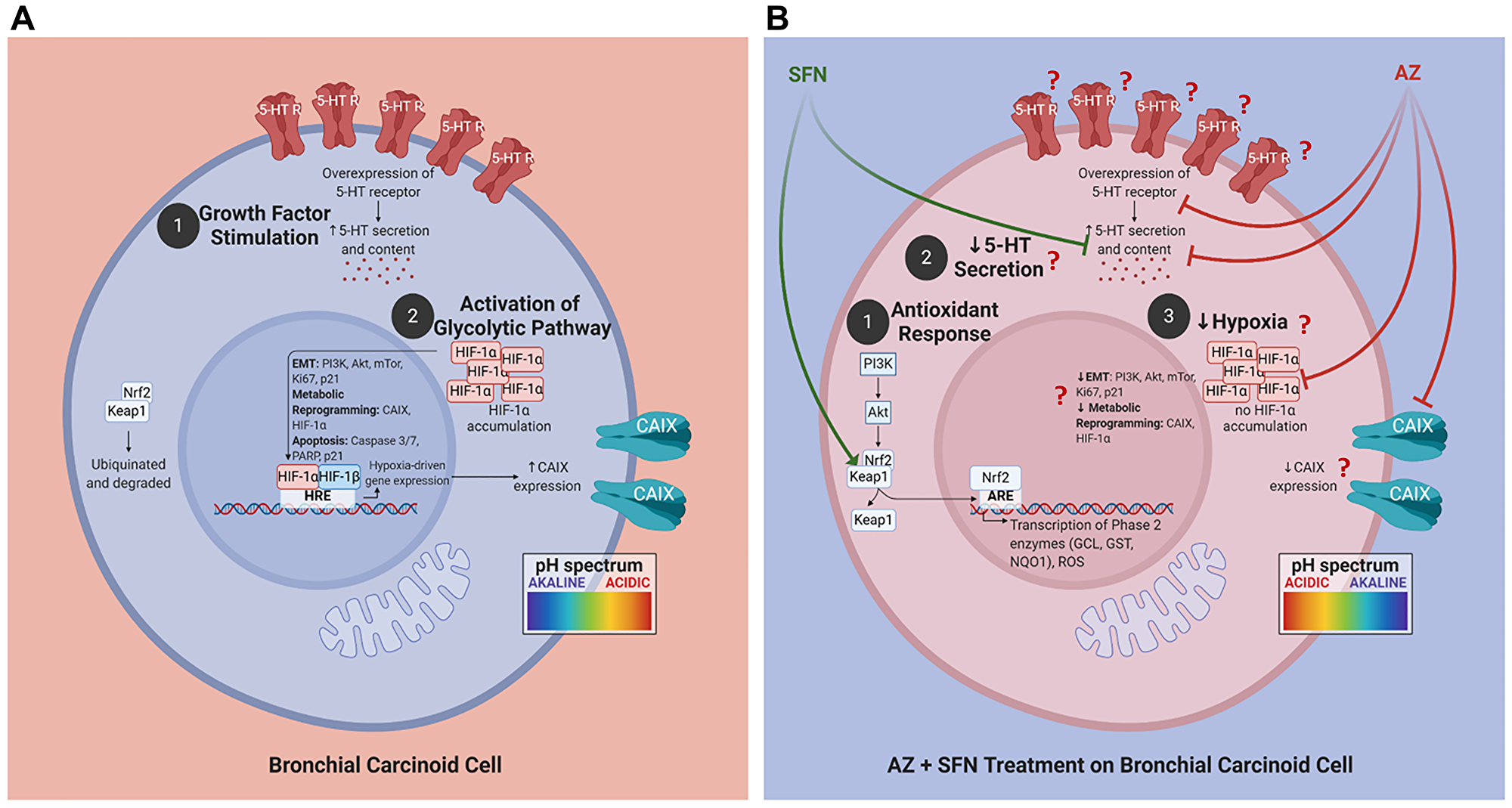
Figure 9: Proposed schema of AZ, SFN, and AZ+SFN targeting the pro-survival pathways in BC. (A) BC cells are in an acidic environment under normal conditions, supporting: (1) growth factor stimulation; and (2) activation of the glycolytic pathway and migratory/invasive potential. (B) AZ+SFN treatment works to: (1) modulate the antioxidant response; (2) decrease 5-HT secretion; and (3) decrease hypoxia, making the cell intracellularly more acidic and the extracellular environment more alkaline. The pan-inhibition of these pathways and critical components can ultimately reduce or abrogate BC cells' tumorigenic potential. The question marks in B require further evidence and study. The images were created with https://biorender.com. Abbreviations: AZ: acetazolamide; SFN: sulforaphane; BC: bronchial carcinoma.
The Yeger/Bayat/Szewczuk Research Team concluded in their Oncotarget Research Output that CAIX has also been identified as a regulator of 5-HT secretion in NETs due to its cytosolic pH homeostatic role in hypoxic tissues.
5-HT is released by BC and other NETs in response to hypoxic conditions, leading to autocrine growth signaling and subsequent systemic symptoms.
These authors have previously shown that AZ, SFN, and AZ SFN successfully reduce 5-HT vesicular content in BC.
SFN acts synergistically to reduce 5-HT receptor expression, thereby blocking growth signaling in tumor cells.
The effectiveness of the combination of AZ and SFN reduces 5-HT content and secretion in H727 and H720 tumor cell xenografts assessed by amperometry.
DOI - https://doi.org/10.18632/oncotarget.28011
Full text - https://www.oncotarget.com/article/28011/text/
Correspondence to - Herman Yeger - [email protected], Reza Bayat Mokhtari - [email protected], and Myron R. Szewczuk - [email protected]
Keywords - sulforaphane, acetazolamide, bronchial carcinoid tumors, serotonin, carbonic anhydrase
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105



