Oncotarget Volume 11 Issue 14 reported that of 80 patients enrolled, common tumors included gastrointestinal stromal tumors, colorectal cancer, and ovarian cancer.
The most common treatment-related adverse events were fatigue, diarrhea, nausea, and vomiting.
In this heterogeneous patient population, the safety profile was acceptable for dovitinib therapy.
A subset of patients with RTK pathway-activated tumors experienced clinical benefit.
Dr. Matthew H. Taylor from the Division of Hematology and Medical Oncology, Knight Cancer Institute, Oregon Health & Science University, Portland, OR, USA said, "Receptor tyrosine kinases (RTKs), including vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and the proto-oncogene cKIT play multiple roles in tumor growth, development, and survival."
"Receptor tyrosine kinases (RTKs), including vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and the proto-oncogene cKIT play multiple roles in tumor growth, development, and survival."
- Dr. Matthew H. Taylor, Division of Hematology and Medical Oncology, Knight Cancer Institute, Oregon Health & Science University
Receptor tyrosine kinases, including vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor, and the proto-oncogene cKIT play multiple roles in tumor growth, development, and survival.
In the clinic, dovitinib has demonstrated promising activity in a number of cancers, including as a single agent in gastrointestinal stromal tumors and in combination with fulvestrant in advanced breast cancer.
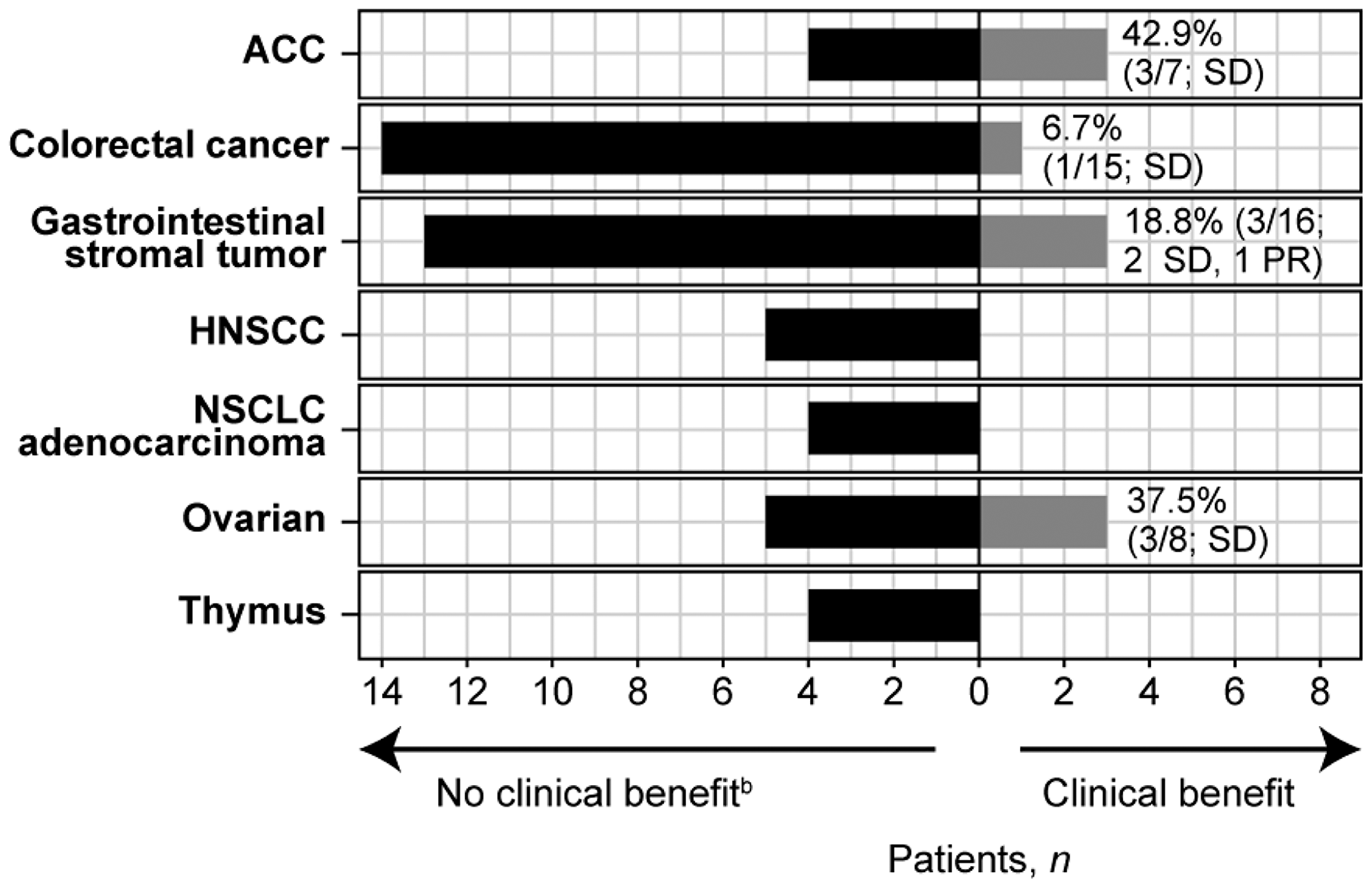
Figure 1: Clinical benefit per tumor type cohorta. aOnly tumor types with at least 4 patients are included. bPatients for whom clinical benefit was non-evaluable are included under "no clinical benefit" as follows: ACC n = 1; Colorectal cancer n = 2; Gastrointestinal stromal tumor n = 3; HNSCC n = 3; NSCLC adenocarcinoma n = 1; ovarian n = 3; thymus n = 3. Abbreviations: ACC, adenoid cystic carcinoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer; PR, partial response; SD, stable disease.
The purpose of this tumor histology-agnostic study was to determine whether dovitinib treatment demonstrated sufficient efficacy in select RTK pathway-activated cancers to support additional studies.
The Taylor Research Team concluded in their Oncotarget Research Article, "dovitinib therapy was well tolerated in this heavily pretreated patient population and clinical benefit was observed in a subset of patients following dovitinib treatment. Future studies may provide additional insights into the role of the tumor genomic background in response to dovitinib therapy."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27530
Full text - https://www.oncotarget.com/article/27530/text/
Correspondence to - Matthew H. Taylor - [email protected]
Keywords - advanced malignancies, basket trial, dovitinib, histology-agnostic, mutation-specific
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105


