Targeted therapies aimed at tumor vasculature are utilized in combination with chemotherapy to improve drug delivery and efficacy after tumor vascular normalization.
The research team's data in mouse models indicate that activation of calcineurin-NFAT-TSP1 signaling in endothelial cells plays a critical role in exercise-induced shear stress mediated tumor vessel remodeling.
They show that moderate aerobic exercise with chemotherapy caused a significantly greater decrease in tumor growth than chemotherapy alone through improved chemotherapy delivery after tumor vascular normalization.
Dr. Sandra Ryeom from the Department of Cancer Biology at the Perelman School of Medicine University of Pennsylvania, Abramson Family Cancer Research Institute, in Philadelphia, PA, USA said, "Tumor vasculature is unorganized and leaky; as many as 50% of tumor vessels are non-functional."
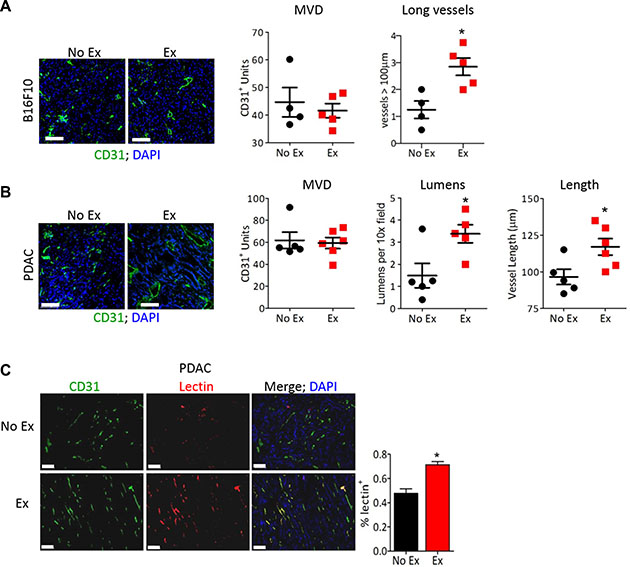
Figure 1: Early-life intervention with ctB rescues mice from age-associated obesity and inflammation. Figure 1: Exercise normalizes tumor vasculature. (A, B) Representative images of anti-CD31 immunofluorescence on (A) B16F10 or (B) PDAC-4662 tumors with DAPI staining to visualize the nucleus. After B16F10 or PDAC-4662 tumors were palpable, mice were randomized into treadmill running or no exercise groups. 21 days later, tumors were harvested. Microvessel density (MVD), the number of vessels > 100 μm (large), the number of visible lumens and the average vessel length, and were counted in 5 random sections/tumor and averaged to obtain a value per tumor, *p < 0.05, n = 5–6 per group, Bars = 100 μm. (C) Representative images of anti-CD31 immunofluorescence and isolectin-B4 positive vessels from PDAC-4662 tumors. When tumors were palpable, PDAC-4662 tumor-bearing mice were treated with gemcitabine with or without daily treadmill running for two weeks. Prior to euthanasia, mice were injected with isolectin-B4. Quantification of Isolectin-B4 (red) and CD31 (green) double positive vessels is shown on the right with double positive vessels quantified in 5 sections/tumor and shown as the mean +/– S.E.M., n = 4 per group, *p < 0.05, Bars = 100 μm.
The concept of vascular normalization, originally proposed by Jain and colleagues in 2001, posits that restoring the balance of angiogenic regulators by decreasing pro-angiogenic factors remodels tumor vasculature to become more organized and functional, similar to normal vasculature.
Thus, the authors reasoned that increasing blood flow in tumor vasculature may promote tumor vessel function.
Recently, tumor vascular remodeling in response to exercise has been described in mouse cancer models, but the molecular mechanisms regulating the tumor vascular response to exercise have not been elucidated.
Aerobic exercise triggered tumor vascular normalization, characterized by increased vascular length and perfusion and increased chemotherapy delivery to the tumor bed.
Utilizing microfluidic devices and modified tissue culture dishes to mimic increased blood flow-induced shear stress in vitro, they found that shear stress alters the angiogenic secretome of endothelial cells, leading to secretion of soluble factors that inhibit tumor EC sprouting and permit vascular remodeling.
Further, the research demonstrates that fluid flow activates the transcription factor Nuclear Factor of Activated T cells in ECs, modulating the expression of multiple pro- and anti-angiogenic factors.
The Ryeom research team concluded, "Moderate intensity treadmill running in mice, roughly the equivalent of brisk walking in humans at approximately 60 70% V02 max, enhanced the efficacy of chemotherapy."
Full text - https://doi.org/10.18632/oncotarget.11748
Correspondence to - Sandra Ryeom
Keywords - tumor vascular normalization, NFAT, thrombospondin-1, exercise


