The cover for issue 69 of Oncotarget features Figure 5, "qRT-PCR analysis of select mRNAs in mTORC1/2 inhibited cells with and without carboplatin treatment," by David-West, et al.
Using platinum-resistant OVCAR-3 cells treated with the selective mTORC1/2 inhibitor INK128/MLN128, the research team conducted genome-wide transcription and translation studies and analyzed the effect on cell proliferation, AKT-mTOR signaling and cell survival, to determine whether carboplatin resistance involves selective mRNA translational reprogramming, and whether it is sensitive to mTORC1/2 inhibition.
Genome-wide transcriptomic and translatomic analyses in OVCAR-3 cells revealed that the modest downregulation of global protein synthesis by dual mTORC1/2 inhibition is associated with greater selective inhibition of DDR, cell cycle and survival mRNA translation, which was confirmed in platinum-resistant SKOV-3 cells.
Dr. Robert J. Schneider from the Department of Microbiology, New York University School of Medicine, New York, NY, USA and the Perlmutter Cancer Center, New York University School of Medicine, New York, NY, USA said, "Ovarian cancer is the second most common gynecologic malignancy, and the number one cause of death among all gynecologic malignancies."
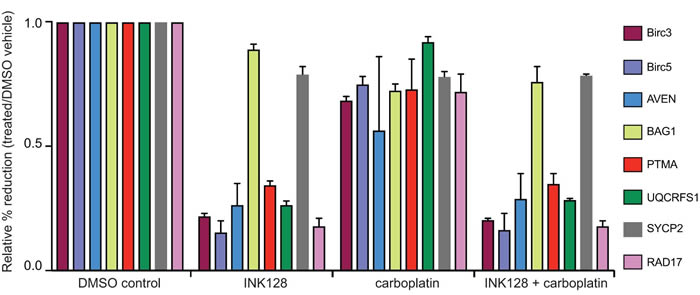
Figure 5: qRT-PCR analysis of select mRNAs in mTORC1/2 inhibited cells with and without carboplatin treatment. Cells were treated at GI50 dose levels with 2 μM carboplatin for 5 h followed by 3 h or 18 h of 0.25 μM INK128 prior to cell harvest. Total mRNA and ≥4 ribosome heavy polysome mRNA was prepared as in Figure 3 legend. The abundance of selected mRNAs that encode proteins in the DNA damage response and survival response were assessed by qRT-PCR of the ≥4 ribosome (heavy polysome) fraction corresponding to well-translated mRNAs compared to total mRNA levels for each corresponding mRNA (see Methods for details). Results shown are the average of three independent experiments.
Specifically, the PI3K/AKT/mTOR pathway is commonly upregulated with increased activation in most subtypes of epithelial ovarian carcinoma, including approximately 50% of high-grade serous ovarian carcinomas. With a significant role in tumorigenesis, including that of ovarian cancer, inhibition of the mTOR pathway has been extensively investigated in the preclinical and clinical settings. MTOR forms two protein complexes with different activities, mTOR complex 1 and mTOR complex 2.
Clinical experience with mTOR inhibitors in ovarian cancer have to date been derived from the use of rapalogs that inhibit only mTORC1 in early stage clinical studies. The research shows that platinum resistance of ovarian cancer cells can be reversed by inhibition of mTORC1/2 and involves the greater translational inhibition of specific mRNAs encoding survival, cell cycle and DDR functions.
The Robert J. Schneider research team concluded, "Using genome-wide transcriptomic and translatomic analyses, we found a selective reduction in translation of specific mRNAs resulting from mTORC1/2 inhibition that sensitizes to genotoxic chemotherapy by impairing translation of mRNAs that promote increased cell survival and DNA damage and repair functions, and possibly mRNA translation functions, all of which are involved in resistance to carboplatin-mediated cell killing. It was particularly interesting that the greatest number of mRNAs that were reduced in translation efficiency were found in the combined treatment set, despite the fact that carboplatin does not directly impair protein synthesis, which was shown to result solely from INK128 inhibition of mTORC1/2. Our data suggest that this is a result of moderately increased expression of mRNAs involved in the DNA damage and repair responses, as well as survival and cell cycle pathways, resulting from carboplatin treatment, whose translation is particularly sensitive to dual mTORC1/2 inhibition."
Full text - https://doi.org/10.18632/oncotarget.25869
Correspondence to - Robert J. Schneider - [email protected]
Keywords - mTOR, ovarian cancer, platinum resistance, translational regulation


