The cover for issue 58 of Oncotarget features Figure 7, "Models of WNT-mediated transcriptional activation and repression involving TCF7L2, AP-1, β-catenin and APC," by Hankey, et al.
The APC protein performs multiple tumor suppressor functions including negative regulation of the canonical WNT signaling pathway by both cytoplasmic and nuclear mechanisms. Published reports that APC interacts with -catenin in the chromatin fraction to repress WNT-activated targets have raised the possibility that chromatin-associated APC participates more broadly in mechanisms of transcriptional control. High-confidence targets additionally validated in mouse adenomas included 16 increased and 9 decreased in expression following APC loss, indicating that chromatin-associated APC may antagonize canonical WNT signaling at both WNT-activated and WNT-repressed targets.
Dr. Joanna Groden from the Department of Cancer Biology and Genetics, The Ohio State University College of Medicine, Columbus, Ohio, United States of America said, "Biallelic APC mutations initiate the development of a high percentage of colorectal cancers."
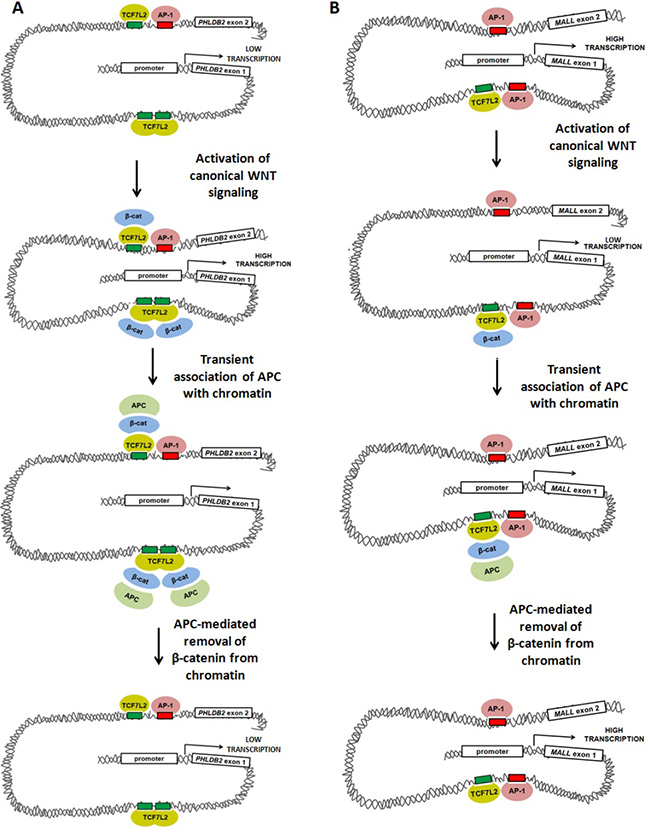
Figure 7: Models of WNT-mediated transcriptional activation and repression involving TCF7L2, AP-1, β-catenin and APC. Our data suggest at least two models in which canonical WNT-mediated transcriptional activity, APC and AP-1 alter gene expression. (A) Activation of canonical WNT signaling up-regulates transcription of PHLDB2 through a previously-characterized mechanism in which β-catenin binds to the TCF7L2 transcription factor, promoting DNA bending that brings transcriptional machinery (including AP-1) into closer association with the proximal promoter. APC disrupts this association by mediating the removal of β-catenin from the complex. (B) Activation of canonical WNT signaling may down-regulate AP-1-dependent transcription of MALL through a mechanism in which β-catenin binding to TCF7L2 disrupts interaction of transcriptional machinery with the proximal promoter. APC may relieve this disruption by removing β-catenin from the complex.
APC interacts with -catenin in a cytoplasmic complex that facilitates -catenin degradation, while nuclear APC facilitates both -catenin export to the cytoplasm and -catenin removal from specific genomic loci. Chromatin immunoprecipitation of APC and next-generation sequencing were performed from HCT-116 colon cancer cells, which express wild-type APC yet can model the APC loss observed in the majority of colorectal cancers following transient si RNA-based silencing. Gene expression data were collected from HCT-116 cells in the presence or absence of si RNA targeting APC and were compared to Ch IP-seq data to identify candidate genes controlled by chromatin-associated APC. High-confidence candidate genes were likely shared targets of canonical WNT signaling and surprisingly included both genes increased in expression following APC loss and decreased in expression following APC loss.
The Joanna Groden research team concluded, "The high rates of co-occurrence of TCF7L2 and AP-1 binding sites observed in the Ch IP-seq data further indicate that the presence or absence of AP-1 binding sites may be an additional criterion by which to sort target genes. The natural product veratramine is an additional small molecule inhibitor of AP-1 that inhibits transactivation by interacting not with AP-1 itself but with its TGACTCA binding motifs."
Full text - https://doi.org/10.18632/oncotarget.25781
Correspondence to - Joanna Groden - [email protected]
Keywords - APC, AP-1, canonical WNT signaling, chromatin, colorectal cancer


